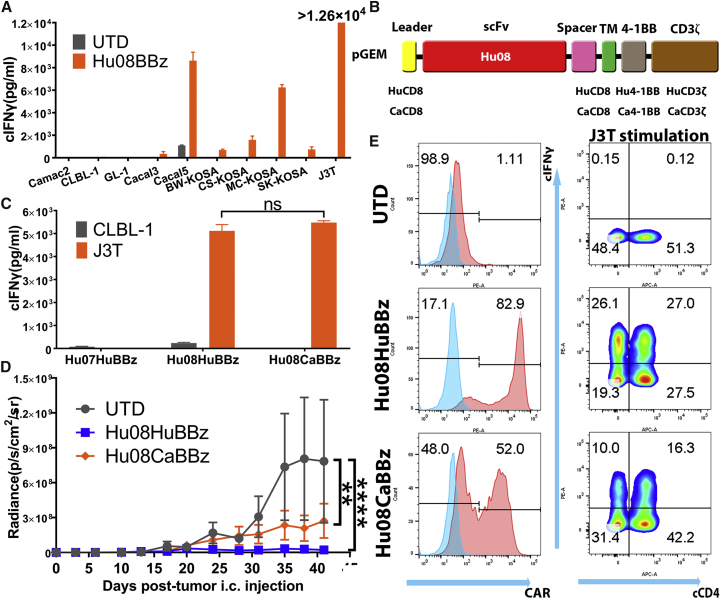
Figure 6.
Canine IL-13Rα2 CAR T Cells Control Canine Tumor Growth
(A) mRNA-electroporated Hu08BBz canine CAR T cells were co-cultured with canine tumor cell lines (Camac2, CLBL-1, GL-1, Cacal3, Cacal5, BW-KOSA, CS-KOSA, MC-KOSA, SK-KPSA, and J3T). Canine IFNγ secretion was detected with ELISA and compared the stimulation with un-transduced canine T cells. (B) Vector maps of anti-IL-13Rα2 human Hu08BBz CAR structure (Hu08HuBBz) and canine Hu08BBz CAR structure (Hu08CaBBz). (C) mRNA-electroporated Hu07HuBBz, Hu08HuBBz, and Hu08CaBBz canine CAR T cells co-cultured with CLBL1 and J3T tumor cell lines. Canine IFNγ secretion was detected with ELISA. Unpaired t test was used to determine the statistically significant difference of IFNγ secretion between Hu08HuBBz and Hu08CaBBz co-cultured with J3T glioma cells. (D) J3T canine glioma cell line orthotopically implanted into the NSG mouse brain. 12 million electroporated Hu08HuBBz, Hu08CaBBz, or un-transduced canine T cells were i.v. injected into the mouse model (n = 4 per group) on days 7, 10, and 13 after tumor implantation. Tumor growth was evaluated by bioluminescence imaging every 3–4 days. Statistically significant differences of tumor growth were determined by linear regression. (E) The canine T cells used on the second injection on day 10 were analyzed for CAR expression and canine IFNγ secretion after co-culture with the J3T tumor cell line. Canine CD4 was stained to distinguish the canine CD4- and CD8-positive subgroups along the x axis. ns, not significant; **p < 0.01, ****p < 0.0001. Data are presented as means ± SEM.