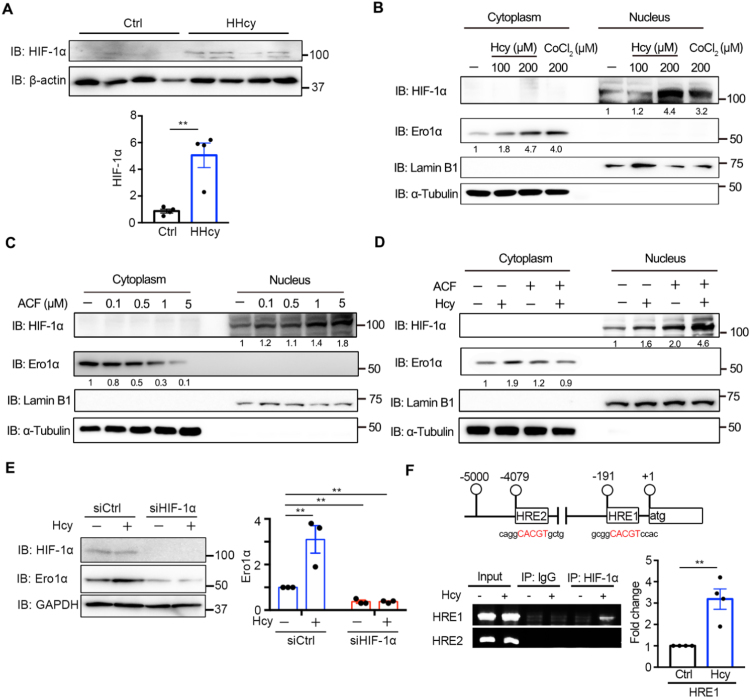
Fig. 3.
Hcy-induced expression of Ero1α is through HIF-1α. (A) Lysates prepared from the thoracic aortas of normal (Ctrl) or HHcy mice were subjected to immunoblotting and statistical analysis for HIF-1α. Data were presented as mean ± SEM from four biological replicates, **p < 0.01 via two-tailed Student's t-test. (B) HUVECs were treated with indicated concentrations of Hcy or CoCl2 for 4 h. Cytosolic Ero1α and nuclear HIF-1α were measured by immunoblotting with α-Tubulin and Lamin B1 as loading controls for cytosolic and nuclear extracts, respectively. (C) HUVECs were treated with different concentrations of ACF for 48 h, and immunoblotting was carried out as in (B). (D) HUVECs were pre-treated without or with 5 μM ACF for 1 h, then treated without or with 200 μM Hcy for an additional 4 h, and immunoblotting was carried out as in (B). (B–D) The relative density of each band was quantified as indicated below, which were representative results from two independent experiments. (E) HUVECs transfected with scrambled siRNA (siCtrl) or siRNA targeting HIF-1α for 72 h were treated with PBS or Hcy for 4 h. Cell lysates were analyzed by immunoblotting and statistical analysis for Ero1α. Data were shown as mean ± SEM from three independent experiments, **p < 0.01 via two-way ANOVA, Tukey's multiple comparisons test. (F) (Upper) Locations of the putative HRE-containing sites within the ERO1A promoter. The sequences that fit the consensus HRE motif were shown in red. (Lower) ChIP analysis of HIF-1α occupancy on the ERO1A promoter was performed with IgG or anti-HIF-1α antibody in HUVECs with or without 200 μM Hcy treatment for 4 h. The putative HRE1 and HRE2 were amplified by PCR and agarose gel electrophoresis. Enrichment values were shown as fold changes normalized to input. Data were presented as mean ± SEM from three independent experiments, **p < 0.01 via two-tailed Student's t-test.