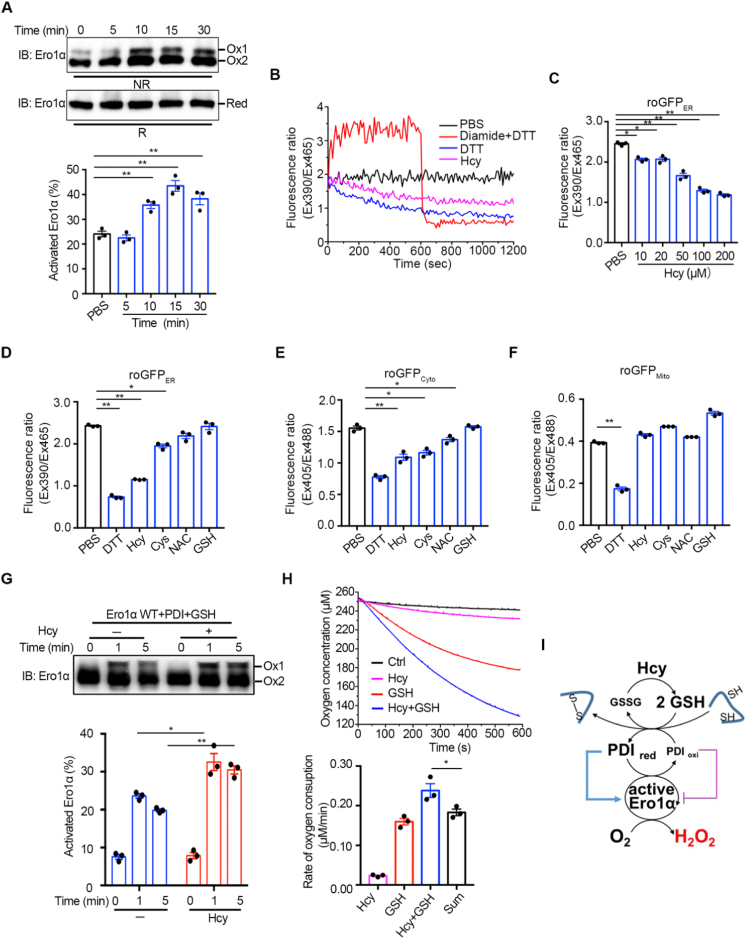
Fig. 4.
Hcy activates Ero1α by reducing the redox states in the ER of HUVECs. (A) (Upper) HUVECs were treated with 200 μM Hcy and quenched with 20 mM NEM at indicated times. The cell lysates were analyzed by nonreducing (NR) or reducing (R) SDS-PAGE, followed by immunoblotting. Two oxidized Ero1α species (Ox1, active form; Ox2, inactive form) and fully reduced Ero1α (Red) were indicated. Representative blots were shown. (Lower) The ratio of activated Ero1α was quantified by densitometry and calculated. (B) HUVECs expressing roGFPER were suspended in HBSS buffer (black) and treated with 0.5 mM diamide for 600 s, followed by the addition of 10 mM DTT (red), 100 μM DTT (blue), or 200 μM Hcy (pink). The ratio of fluorescence intensity was traced. (C) Measurement of roGFPER fluorescence in HUVECs treated by PBS or different concentrations of Hcy for 30 min. (D–F) HUVECs transfected with roGFPER (D) roGFPCyto (E), or roGFPMito (F) for 48 h were resuspended in HBSS buffer before treated with PBS or different reducing agents at 200 µM thiols as indicated for 30 min and then the fluorescence intensity ratio was detected. (A, C-F) Data were shown as mean ± SEM from three independent experiments, *p < 0.05 and **p < 0.01 via one-way ANOVA, Tukey's multiple comparisons test. (G) (Upper) Ero1α protein at 1 µM was incubated with 10 µM PDI protein and 1 mM glutathione in the presence or absence of 200 μM Hcy for a different time as indicated, and then analyzed by nonreducing SDS-PAGE and immunoblotting. Representative blots were shown. (Lower) The ratio of activated Ero1α were quantified as in (A). Data were shown as mean ± SEM from three independent experiments, *p < 0.05 and **p < 0.01 via two-way ANOVA, Tukey's multiple comparisons test. (H) (Upper) Oxygen consumption catalyzed by 2 µM Ero1α was monitored in the presence of 20 µM PDI without (black) or with 400 μM Hcy (pink), 2 mM GSH (red) or 400 μM Hcy plus 2 mM GSH (blue). (Lower) The slopes of the linear phases of oxygen decrease were taken as the oxygen consumption rates. The oxygen consumption rate in the control group (black) was taken as background and was subtracted in each group. The sum of the oxygen consumption rates in the presence of 400 μM Hcy or 2 mM GSH alone was taken as ‘Sum’. Data were shown as mean ± SEM from three independent experiments, *p < 0.05 and **p < 0.01 via one-way ANOVA, Tukey's multiple comparisons test. (I) Schematic model illustrating that Hcy increases GSH/GSSG in the ER, therefore activates Ero1α and leads to H2O2 production.