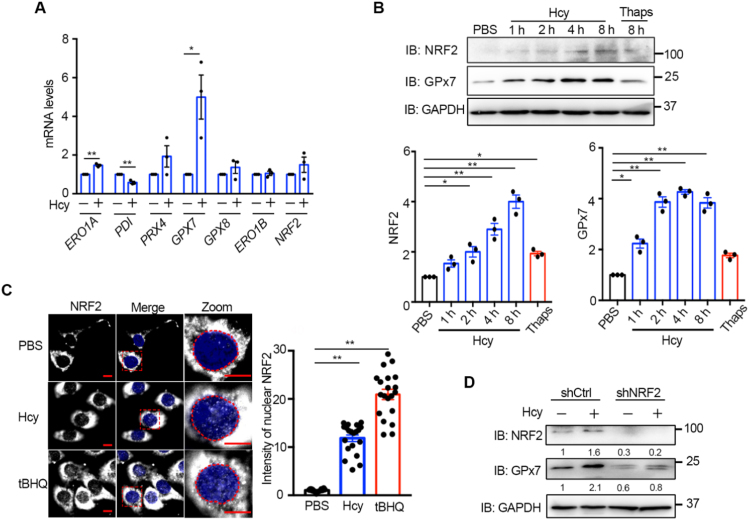
Fig. 6.
Hcy activates the NRF-2/GPx7 pathway in HUVECs. (A) RT-qPCR analysis of ER redox-related genes in HUVECs treated without or with 200 μM Hcy for 8 h. All values were normalized to β-ACTIN mRNA. Data were represented as mean ± SEM from three independent experiments each performed in three technical replicates, *p < 0.05 and **p < 0.01 via two-tailed Student's t-test. (B) HUVECs were treated with 200 μM Hcy for different times as indicated or with 5 μM Thaps for 8 h. The cell lysates were subjected to immunoblotting and statistical analysis for NRF2 and GPx7 expression. Representative blots were shown. Data were represented as mean ± SEM from three independent experiments. *p < 0.05 and **p < 0.01 via one-way ANOVA, Tukey's multiple comparisons test. (C) Immunofluorescence analysis of the translocation of NRF2 (Ex = 488 nm) into the nucleus (blue, Ex = 405 nm) in HUVECs treated with 200 μM Hcy or 200 μM tertiary butyl hydroquinone (tBHQ) or PBS for 2 h. The nuclei were outlined with red circles. Scale bars, 10 µm. The relative fluorescence intensities from nuclear-localized NRF2 were quantified. Data were represented as mean ± SEM, N = 20 cells, **p < 0.01 via one-way ANOVA, Tukey's multiple comparisons test. (D) HUVECs transduced with lentiviral shCtrl or shNRF2 for more than 72 h were treated without or with 200 μM Hcy for 4 h. The protein levels of NRF2 and GPx7 were determined by immunoblotting and quantified by densitometry as indicated below, which were representative results from two independent experiments.