Abstract
The unparalleled biodiversity found in the American tropics (the Neotropics) has attracted the attention of naturalists for centuries. Despite major advances in recent years in our understanding of the origin and diversification of many Neotropical taxa and biotic regions, many questions remain to be answered. Additional biological and geological data are still needed, as well as methodological advances that are capable of bridging these research fields. In this review, aimed primarily at advanced students and early-career scientists, we introduce the concept of “trans-disciplinary biogeography,” which refers to the integration of data from multiple areas of research in biology (e.g., community ecology, phylogeography, systematics, historical biogeography) and Earth and the physical sciences (e.g., geology, climatology, palaeontology), as a means to reconstruct the giant puzzle of Neotropical biodiversity and evolution in space and time. We caution against extrapolating results derived from the study of one or a few taxa to convey general scenarios of Neotropical evolution and landscape formation. We urge more coordination and integration of data and ideas among disciplines, transcending their traditional boundaries, as a basis for advancing tomorrow’s ground-breaking research. Our review highlights the great opportunities for studying the Neotropical biota to understand the evolution of life.
Keywords: Biogeography, Biotic diversification, Landscape evolution, Phylogeny, Scale, Biodiversity, Community ecology, Phylogeography, Phylogenetics, Tropics
Introduction
The Neotropical region (also referred to as tropical America or the American tropics) extends today from central Mexico to Argentina, including the Caribbean (Morrone, 2013). The region encompasses a vast range of biomes and habitat types, each with a particular history of landscapes and biotic evolution (Fig. 1; Hughes, Pennington & Antonelli, 2013). For many groups of organisms, the Neotropics are home to outstandingly high levels of species richness, when compared to other major biotic realms (Lundberg et al., 2000; Antonelli & Sanmartín, 2011). As such, understanding Neotropical biodiversity patterns and the processes associated with its origin and maintenance represents a major scientific priority.
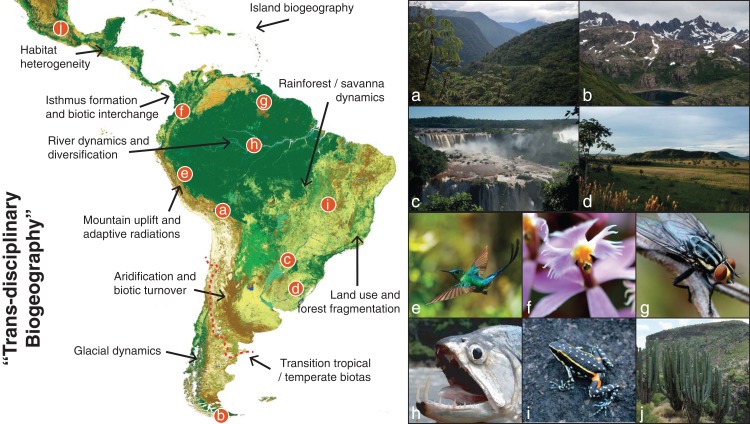
Figure 1. The giant Neotropical puzzle.
Map of the Neotropical region, spanning from Central Mexico to central Argentina (red dashed line) and including all Caribbean Islands. The figure shows examples of the large diversity of Neotropical habitats and the taxa that inhabit those habitats. We also outline a few of the many topics in Neotropical biodiversity that can be studied in the “trans-disciplinary biogeographic approach” advocated here. (A) Eastern slopes of the Bolivian Andes, where the Amazonian and Andean biotas meet; (B) Patagonian mountains of southern Chile, which despite being in the temperate zone of South America is home to many Neotropical-derived lineages; (C) Iguazu waterfalls, where increased humidity create gallery forests within the South American open diagonal; (D) Southern grasslands of the Pampas, a naturally open habitat now largely influenced by human activity; (E) One of the ca. 338 known species of hummingbirds, a conspicuous clade currently restricted to the American continent and particularly diverse in the Andes; (F) Epidendrum ibaguense, a widespread species in the orchid family in which many new Neotropical species are discovered each year; (G) An unidentified fly in the inselbergs of southern French Guiana, where basaltic rocks emerge several hundred meters above the surrounding Amazonian rainforest; (H) The large dogtooth characin fish Hydrolycus scomberoides, exemplifying the world’s richest ichthyofauna in the Amazon drainage basin; (I) Ameerega flavopicta, a rock-dwelling frog species adapted to a region of high seasonality of precipitation; (J) A columnar cactus of central Mexico, near the northwestern limits of the Neotropical region where low-canopy forests and succulent vegetation build vegetation mosaics across the landscape. Map generated through the remote-sensing ESA GlobCover 2009 project and colored by biome assignments (©ESA 2010 and UCLouvain; http://due.esrin.esa.int/page_globcover.php). Photo credits: A–G, I and J: A.A.; H: J.A.
Biodiversity refers to the diversity of life across all levels of biological organization (Purvis & Hector, 2000; Gaston & Spicer, 2004). Biodiversity is unevenly distributed across Earth, and varies among and within geographic regions, between terrestrial and aquatic ecosystems, and among different groups of organisms. Biodiversity increases from the poles to the equator, reaching the highest values in tropical regions, a pattern termed the latitudinal diversity gradient (Willig, Kaufman & Stevens, 2003; Field et al., 2009). However, this pattern is complex, with numerous non-diverse tropical or diverse non-tropical areas and taxa constituting exceptions. In addition, there are still numerous uncertainties in the underlying data used to generalize overall patterns. Most importantly, these patterns remain far from properly understood, and we are still struggling to identify their main determinants. As a result, researchers tend to focus on different aspects of biodiversity such as taxonomic, phylogenetic, and functional diversity (FD) (Swenson, 2011; Tables 1–3). Each of these aspects of biodiversity may vary among regions and taxa, and each must therefore be assessed by independent criteria (Strecker et al., 2011; Magurran, 2013).
Table 1. The concept of taxonomic diversity, and its use and challenges in the Neotropical context.
| Definition. Taxonomic diversity refers to how many taxa can be found within a given area or higher clade, and how individuals are distributed among these taxa. Taxonomic diversity can be quantified at different taxonomic ranks (e.g., species, genera, families), with the species rank being the most popular by far. Species richness is widely viewed as a fundamental measure of overall biodiversity (Gotelli & Colwell, 2001). This is due to the fact that the species boundary defines the limits of genetic variation, natural selection, and adaptation (Sexton et al., 2009). While individual organisms live and die, the stable phenotypes recognized as species may persist for millions of years, serving as predictable components of the ecosystems in which all species function and evolve (Eldredge, 1989). As result, species are thought to constitute the basic structural and functional units in ecology and evolution (Tilman & Downing, 1994; Worm et al., 2006). |
| Generic and family-level taxonomic ranks are occasionally used in comparative studies, especially when species identification or delimitation is difficult (Bertrand, Pleijel & Rouse, 2006). However, the ranks that taxonomists must assign to higher-level taxa are often considered to be arbitrary constructs, reflecting little biological organization, and incorporating further biases and artefacts when compared, although opposing views exist (Humphreys & Barraclough, 2014). In general, species are seen as the “fundamental category of biological organization” despite the multitude of species definitions available (De Queiroz, 2005). |
| Metrics and usage. Taxonomic diversity is most commonly measured using taxon richness, that is, the number of taxa in a given area. However, relative abundance distributions can differ greatly among areas, and an area where taxon abundances are equal has intuitively higher diversity than an area with the same number of taxa but a high degree of dominance by one or a few taxa. Abundance differences can be taken into account by quantifying diversity as the effective number of species (also known as Hill number or true diversity; Hill, 1973; Jost, 2006; Tuomisto, 2010, 2018). Because its values are easier to interpret and compare than those of traditional diversity indices (e.g., Brillouin, Shannon, and Simpson indices), the effective number of species is emerging as the best general measure of diversity by a broad consensus. Quantitative abundance data are rather rare, though, and few studies have included abundance when discussing diversity in the Neotropics (but see Valdujo, Carnaval & Graham, 2013; Ter Steege et al., 2013; Tuomisto, Zuquim & Cárdenas, 2014; Jenkins et al., 2015; Moura et al., 2016; Azevedo, Valdujo & Nogueira, 2016; Gómez et al., 2018). |
| Observed taxonomic diversity is sensitive to sampling effort, especially at the species rank. Since communities typically contain many species that are locally rare, observed species richness provides only an underestimate of the number of species actually present, unless the community is very thoroughly sampled. The accuracy of estimates of taxonomic diversity depends on the number of individuals sampled, the size of the local species pool, the evenness of species abundances in the community, size and environmental heterogeneity of the area, and the status of taxonomic knowledge of the groups surveyed. When comparing estimates of local taxonomic diversity among areas, it is therefore important that they are based on quantitative and standardized sampling (Chao & Jost, 2012; Tuomisto, 2018). |
| Beta diversity and species turnover, reflecting heterogeneity in species composition among sites, are also of interest (Tuomisto, 2010, 2018; Higgins et al., 2011; Leprieur et al., 2011; J. M. Craig et al., 2018, unpublished data). However, quantifying these requires data where species identifications have been done consistently using a standard taxonomy, and such data are only available in some areas for some vascular plants (e.g., trees, ferns; Tuomisto, Ruokolainen & Yli-Halla, 2003; Arellano et al., 2016; Tuomisto et al., 2016), and some vertebrates (e.g., birds, primates, some fishes; Arrington & Winemiller, 2009). For these same organisms, a general understanding of species richness gradients has emerged (Kier et al., 2005; Albert et al., 2011; Rosauer & Jetz, 2014; Tuomisto, Zuquim & Cárdenas, 2014). For most other organisms, too few data are available to allow accurate circumscriptions of taxa and reasonable estimates of species richness gradients and species turnover (Andújar et al., 2015). Indeed, the smaller and less conspicuous the organism, the poorer the state of knowledge. For instance, very little is known about microbial and fungal diversity, and insect diversity is similarly understudied (Basset et al., 2012). However, even among well-studied and charismatic Neotropical taxa—such as birds and mammals, even river dolphins—there are still new species to discover (Hrbek et al., 2014). |
Table 3. The concept of functional diversity, and its use and challenges in the Neotropical context.
| Definition. Functional diversity (FD) measures differences in the physiological, behavioral, and ecological characteristics of organisms, and how biological trait values (such as body mass of animals, and life form or habit of plants) affect ecological and evolutionary processes. Knowledge about species traits and ecological functions (such as a species trophic level, and including the variation in traits within and among species) is a crucial component of biodiversity. However, this is one of the major shortcomings in current biodiversity knowledge, especially in tropical areas. Few studies to date have mapped large-scale patterns of functional diversity, although efforts in this direction are underway (see for fishes e.g., Arbour & López-Fernández, 2014; Toussaint et al., 2016). |
| Metrics and usage. Apart from the lack of data, the theory behind functional diversity is not yet well consolidated. We still do not know which traits are ecologically and evolutionarily important for different groups, how to compare traits for different sets of organisms, and how functional diversity affects ecosystem productivity, stability, and resilience, especially in the tropics. An additional shortcoming is associated with biotic interactions. Apart from basic information on pollination and dispersal syndromes, we know surprisingly little about most biotic interactions. Very few species interaction networks are available to date (Toju et al., 2017). |
Survey Methodology
In April 2017, we gathered scholars from several countries and scientific backgrounds to discuss Neotropical biodiversity during the “Origins of Biodiversity Workshop” organized by Chalmers University of Technology and the University of Gothenburg (Sweden), under the auspices of the Gothenburg Centre for Advanced Studies. We spent one week outlining the topics presented here, focusing primarily on recent advances and the future of the field. We continued to work remotely toward the conclusion of this publication. The overall goal of this review is to summarize the knowns and unknowns about Neotropical biodiversity, with focus on terrestrial taxa and ecosystems, and to discuss the many opportunities and challenges of this research field. We acknowledge that the wide breadth of the topics discussed here are in part due to this being a summary of ideas produced at a workshop of diverse participants, and that each topic contains a depth that cannot be simply synthesized. For instance, more comprehensive reviews on the theories proposed to explain the origin of Neotropical diversity can be found elsewhere (Moritz et al., 2000; Antonelli & Sanmartín, 2011; Leite & Rogers, 2013; Table 4). We focus here on topics that we think require further development within this research field.
Table 4. Some of the many theories proposed to explain the high levels of (Neo)tropical biodiversity.
| Theory | Key proponent(s) | Summary | Comment |
|---|---|---|---|
| Riverine barrier hypothesis | Wallace (1889) | The formation of large lowland Neotropical rivers like the Amazon resulted in genetic isolation and speciation in taxa ecophysiologically restricted to non-flooded rain forests. | Dynamic river capture is even more effective at isolating and reuniting populations than is the static geometry of dendritic river basins. |
| Pleistocene refugium theory | Haffer (1969) | Most Amazonian birds, and probably other taxa, originated recently in response to Pleistocene climate changes. The repeated contraction of forests in relation to savannas led to the isolation of populations and inability of breeding once they came into secondary contact during inter-glacials. | From initial support for plants and other taxa, this theory has been heavily criticized based on lack of geophysical evidence for savanna expansions, old divergence times from phylogenies, etc. |
| Time-area integrated hypothesis | Fine & Ree (2006) | Diversity can be predicted by the amount of time that species spend in a region, multiplied by the total area of that region. | A modification of this model is a strong predictor of dispersal events across the Neotropics (Antonelli et al., 2018). |
| Phylogenetic niche conservatism | Wiens & Donoghue (2004) | Tropical biotas are more diverse because many lineages of the modern biosphere evolved in the super-greenhouse world of the Mesozoic and early Cenozoic 140–50 Ma and remained in their original environment. | Most clades have origins in warm and wet tropical climates. Most clades at higher latitudes adapted to cold and dry conditions in the Neogene and Quaternary. |
| Out of the Tropics | Jablonski, Roy & Valentine (2006) | Tropical biotas are more diverse because lineages have higher speciation rates, lower extinction rates, and higher net emigration over immigration than lineages in extra-tropical regions. | This is just one popular theory among several others attempting to explain the latitudinal diversity gradient in species richness. |
| Metabolic theory of ecology | Brown & Svenning (2013) | Higher metabolic rates translate into higher rates of speciation and extinction at low latitudes. | Incompletely developed mechanistic links between kinetics at the metabolic, ecophysiological, and evolutionary scales. |
| Tropical productivity | Gillman et al. (2014) | Species richness is positively correlated with net primary productivity because larger populations are less likely to stochastically fluctuate to a population size of zero, which is a sticky boundary. | Metanalyses have shown a unimodal relationship is more common than a monotonic between productivity and species richness (Fraser et al., 2015). |
| Sea‐level fluctuations | Nores (2002) | Repeated sea-level rises during the late Cenozoic led to the allopatric speciation of Amazonian species in true islands. | Model based on current topography, lacking other geophysical evidence. |
| Museum hypothesis | Stebbins (1974) | Tropical lowlands act as “museums” of diversity, in which species of different origins gradually accumulate. | The Neotropics is now considered both “museum” and “cradle” of diversity (McKenna & Farrell, 2006). |
What Do We Know About Patterns of Neotropical Biodiversity?
Taxonomic diversity
Relatively good estimates of taxonomic diversity are only available for well-studied Neotropical taxa, as in other parts of the world. These estimates have been used to identify the best predictors of diversity at large scales (Jenkins et al., 2015; Moura et al., 2016). Although sampling across taxa is comparable or even greater in the Neotropics than in other tropical regions (Fig. 2; Table 1), taxonomic diversity is generally underestimated within the Neotropics, especially for poorly sampled organisms such as fungi, invertebrates, and micro-organisms.
Figure 2. Taxonomic sampling across the world’s tropics.
Density maps for geo-referenced species occurrences available from the Global Biodiversity Information Facility for (A) Mammals, (B) Amphibians, (C) Fishes, (D) Vascular plants between the Tropics of Cancer and Capricorn (23.5°S–23.5°N), showing the main spatial biases of taxonomic sampling. All datasets were cleaned for automatically detectable errors using SpeciesGeoCoder (Töpel et al., 2016). The figure is shown on a cylindrical equal area projection with standard parallels of 11.75°S and 11.75°N. The width of each cell is consistently 1°, while the height of each cell is 1° at the standard parallels, slightly lower at the equator and slightly higher at the Tropics of Cancer and Capricorn. Colors indicate 10-based logarithm of the number of records.
Several examples of species-rich, yet incompletely-documented faunas are available, including large clades of freshwater fishes, amphibians, and some groups of reptiles. Although about 5,600 species of freshwater fishes are currently known in the Amazon, the Orinoco, and adjacent river basins of tropical South and Central America, more than 100 new species are described every year (Van Der Sleen & Albert, 2017). In other words, approximately two new species are described per week, although an even higher number of new species would be expected if more trained taxonomists were available. This rapid pace of species description is not slowing down, and recent estimates for the total number of Neotropical freshwater fishes exceed 8,000 species (Reis et al., 2016). This estimate is remarkable, implying that more than 2,400 fish species might remain to be described in the Neotropics alone, a number that exceeds the combined number of rodent species currently known on Earth. This large number of expected, but still hidden, lineages represents an example of the “known unknowns” of Neotropical biodiversity (Table 5), which may have different underlying explanations (such as lack of taxonomic training, uneven distribution of resources across taxa and habitats, morphological complexity, among others).
Table 5. The various components of Neotropical biodiversity, examples of major aspects known about them, and some of the key topics that remain to be understood.
| Biodiversity components | Known knowns | Known unknowns | Unknown unknowns |
|---|---|---|---|
| Taxonomic diversity | Approximate species numbers for macroscopic organisms; human impacts tend to decrease overall diversity | Large portions of biodiversity are unexplored (i.e., microbes, invertebrates, fungi) | Taxonomic units used in biodiversity studies may not represent comparable ecological or evolutionary units |
| Genetic variation (within species) | Patterns of genetic variation known for very selected taxa | Overall patterns of genetic variation | How generalizable are conclusions drawn by such limited patterns of genetic diversity |
| Phylogenetic diversity | General understanding of the tree (or network) of life | Drivers of diversification | Potential biases in phylogeny reconstruction and time-calibration |
| Spatial patterns of diversity | Hotspots and general patterns of species richness and diversity; broad species ranges for charismatic taxa | Areas of endemism; known patterns of biodiversity are biased; ecological preferences of species; drivers of diversity | Human impact to overall spatial patterns |
| Functional diversity | Large scale productivity patterns | Biotic interactions | Relevance of current functional diversity measures; equivalency in functional traits; relationship between current and future functional diversity |
Current knowledge of taxonomic limits of Neotropical amphibians and reptiles is gradually improving. Several molecular studies have detected high levels of cryptic diversity, that is, the existence of two or more lineages within a known species (Bickford et al., 2007), indicating that the known taxonomic diversity is still underestimated in many orders (Funk, Caminer & Ron, 2011; Fouquet et al., 2012). Even in the much more densely sampled and well-studied Atlantic Rainforest of Brazil, charismatic species of frogs are still being discovered. For instance, seven new species of Brachycephalus were recently described for this region (Ribeiro et al., 2015). Likewise, intraspecific analyses of Neotropical lizards show that the occurrence of cryptic diversity is often manifested across biomes. This subdivision of broadly distributed taxa into multiple cryptic species with more restricted geographic distributions increases the perception of biological diversity of a given region, and has numerous implications for biogeography (Werneck et al., 2012b) and conservation (Simões et al., 2014).
For plants, a quantitative assessment on the discovery of Amazonian trees during the last three centuries was compiled by Ter Steege et al. (2016), showing clear peaks in herbarium collections and new species descriptions. Although the data show a drop in the collection of unknown taxa after the 1980s (Ter Steege et al., 2016), there are still enormous discoveries to be made. For example, in a few years of increased collection efforts, the Guide of the Ducke Reserve (Brazil; Ribeiro, 1999), which covers one of the most thoroughly studied areas of Amazonian forest, increased the number of known vascular plants from 825 (Prance, 1990) to 2,079 (Hopkins, 2005).
One difficulty in assessing taxonomic diversity is that taxonomic units may vary according to the preference of the taxonomist revising a particular group (e.g., whether a “splitter” or a “lumper”), and by the data and methodologies underlying taxonomic revisions and species circumscriptions. This issue becomes obvious when taxonomic treatments of the same group are produced by different researchers independently. For example, the Neotropical palm genus Attalea included 29 species in one monograph (Henderson, Galeano & Bernal, 1995), and 65 species in another taxonomic treatment published just 4 years later (Glassman, 1999). Similarly, the Caribbean palm genus Coccothrinax included 14 species in one taxonomic treatment (Henderson, Galeano & Bernal, 1997) and 53 species in another (Dransfield et al., 2008). Personal preferences to “lumping” vs. “splitting” among taxonomists may have large consequences for biodiversity estimates and have been shown to strongly affect diversification rate estimates (Faurby, Eiserhardt & Svenning, 2016). Taxonomic practices should therefore be considered when comparing taxonomic biodiversity at any scale, and whenever adequate, researchers should take advantage of explicit and reproducible criteria for species delimitations.
Besides lumping vs. splitting, species lists may vary among authorities depending on the inclusion criteria, such as whether or not to include rare occurrences of a species common elsewhere, and how to classify the life forms of species (e.g., primarily herbaceous plants rarely recorded as trees). For large regions such as Amazonia, these are some of the reasons why the number and contents of species lists may differ substantially (Cardoso et al., 2017; H. Ter Steege et al., 2018, unpublished data).
Phylogenetic diversity
Many Neotropical clades are known from just one or a few species that may represent relictual survivors of ancient and otherwise extinct groups (Table 2). This phenomenon is known from most organism groups, from Neotropical fishes (Albert et al., 2011) to plants (Wilson et al., 2012). To study how differences in diversity arise among taxa, some researchers have turned their attention to the study of early-branching, low-diversity clades. Examples of such clades include the leafy cacti (Pereskia and Leuenbergeria spp.; Cactaceae), the South American lungfish (Lepidosiren paradoxa; Lepidosirenidae), the hoatzin (Opisthocomus hoazin; Opisthocomidae), and the coral pipe snake (Anilius scytale; Aniliidae). In contrast, other species are members of species-rich Neotropical clades still in the full bloom of their diversification, like the lianas of tribe Bignonieae with more than 400 species (Lohmann & Taylor, 2014), palms with over 730 species (Dransfield et al., 2008), armoured catfishes (Loricariidae) with 680 species (Armbruster, Van Der Sleen & Lujan, 2018), and tanagers (Thraupidae) with 371 species (Burns, Unitt & Mason, 2016).
Table 2. The concept of phylogenetic diversity, and its use and challenges in the Neotropical context.
| Definition. Phylogenetic diversity (PD) assesses cumulative evolutionary distinctiveness within and among areas and taxa. We do not contest the usefulness of species as entities in the assessment of biodiversity patterns, conservation, and many other disciplines, from medicine to bioengineering. However, species are not universally comparable units, given differences in species concepts, operational criteria of delimitation, and circumscriptions among areas, taxa, and taxonomists. In addition, species differ widely in their evolutionary ages, geographic distributions, habitat tolerances, and degree of genetic structure. Species also differ in the biological attributes of their constituent organisms, and therefore, in the effects that these traits may have on ecological and evolutionary processes. Furthermore, species are really just the tips of larger phylogenetic trees evolving through time. PD is therefore a useful measure to directly compare the degree of phylogenetic divergence among groups and regions. |
| Metrics and usage. The basic idea of PD is to measure the total amount of lineage evolution through time (i.e., branch length) observed among all members of a clade or area (Faith, 1992). Overall, PD has been shown to provide a better estimate of “feature divergence” than species richness alone (Forest et al., 2007), because PD multiplies the species (tips of the tree) and multi-species clades (tree branches with multiple tips) by their phylogenetic age, usually measured in millions of years. However, there are many ways of deriving and applying such metrics from phylogenies. As such, researchers should try to choose the most appropriate index for each situation, as well as acknowledge these differences in cross-taxonomic comparisons (Tucker et al., 2016). Phylogenetic diversity (or more explicitly: divergence), although not “visible” constitutes a potentially powerful concept for increasing the standardization of biodiversity analyses, the recognition of areas for conservation, and our understanding of evolutionary history of clades, among others. |
| Complementary to phylogenetic diversity based on the relationships among taxa, patterns of genetic variation within species also represent a vital but often under-appreciated component of biodiversity. Knowledge of intraspecific genetic variation may also improve the prediction of a species ability to adapt to changing climates, as well as improving understanding of the process of speciation. This type of information is particularly important in the light of ongoing anthropogenic climate change. However, our current knowledge of species genetic diversity is restricted to a few selected species, and overall patterns of intraspecific genetic diversity remain poorly understood. Even among well-studied groups (e.g., mammals), spatial patterns of genetic diversity are effectively unknown within the tropics. |
The first attempts to map phylogenetic diversity (PD) over continental and global scales were conducted for select vertebrate groups for which phylogenies were available and for which distribution patterns are relatively well known, such as amphibians, birds, and mammals (Safi et al., 2011). Other than these, large-scale phylogenetic and FD studies with focus and dense sampling in the Neotropics are scarce. Some progress has been made in mapping PD patterns in the Neotropics for specific clades (Lehtonen et al., 2015; Lovejoy et al., 2010; Rossatto, 2014; Fenker et al., 2014) or at the intraspecific level in the search for areas of high phylogeographic diversity and endemism (Carnaval et al., 2014; Smith et al., 2017; Melo et al., 2018). Several ongoing studies by independent research groups are now working to broaden our knowledge on the spatial distribution of Neotropical PD.
Biases and Gaps in Neotropical Biodiversity Knowledge
There are two main sorts of biodiversity biases and gaps: taxonomic (also called the “Linnaean shortfall”) and spatial (the “Wallacean shortfall”). Taxonomically, detailed information on the richness and geographical distribution of species is restricted to certain well-studied taxa (e.g., primates and birds). We also know much more about organisms on land than those living in freshwater systems, including lakes, rivers, and swamps, leaving a large gap in the knowledge of Neotropical aquatic diversity. Spatially, our knowledge is concentrated to few well-studied areas (e.g., La Selva in Costa Rica, Barro Colorado Island in Panama, the Ducke Reserve in Brazil, Manu National Park in Peru, Yasuni National Park in Ecuador). Some regions stand out as having the lowest levels of sampling, including some parts of Central America, the central Andes, the Caatinga, and large parts of Amazonia (Fig. 2), where we have almost no occurrence records available (Hopkins, 2005; Feeley, 2015; Ter Steege et al., 2016; Tedesco et al., 2017). Clearly, for the vast majority of taxa, regions, and ecosystems, biodiversity knowledge is still scarce.
In general, knowledge of species distributions and diversity patterns are strongly biased toward areas that are more easily accessible by roads, rivers, and research stations (Hopkins, 2005; Meyer et al., 2015). Interestingly, at least Amazonian trees, there seems also to exist a bias toward reporting rare species (Table 6), as most scientific collectors tend to over-collect rare or uncommon trees (Ter Steege et al., 2011), although this pattern may not exist for other taxa. Although bioinformatic solutions may now assist in cleaning, predicting, and validating species occurrence data, taxonomic expertise is still essential but limited (Maldonado et al., 2015; Töpel et al., 2016). As a result of our limited knowledge on species distributions patterns, and large gaps in knowledge about climatic and edaphic conditions for large portions of the Neotropics, the ecological requirements for most species remain unknown (Table 5).
Table 6. The commonness of rarity in Neotropical diversity.
| Most Neotropical species are rare, narrowly distributed, and endemic to particular regions or biomes (see Albert et al., 2011 for fishes; Ter Steege et al., 2016 for plants). Species with low abundances and narrow geographic ranges, as well as those confined to special habitats or areas, represent a sizable portion of Neotropical diversity. Indeed, a recent study extrapolating population size for Amazonian trees suggests that most species in the region are represented by comparatively few individuals (Ter Steege et al., 2013). Another study suggests that a considerable fraction of the rare species in the region may actually have relatively large distribution ranges (Zizka et al., 2017). However, many apparently widespread species in most taxonomic groups have been shown to contain multiple phylogenetic species, a possibility that remains poorly explored in the Neotropics but has important consequences for our understanding of diversity patterns and conservation priorities (Bickford et al., 2007; Colli et al., 2016). The contributions of rare species to diversity patterns are difficult to quantify and remain largely obscure (Coddington et al., 2009), partly because most truly rare species will be completely unknown, and partly because rareness in the ecological sense is hard to define, depending on a variety of aspects, including the species concept adopted and the taxonomic preferences. |
| In both fish and plant taxa, areas of endemism separated by prominent biogeographic barriers, such as Amazonian and Mesoamerican rainforests currently separated by the Andes, arise from dispersal limitation, and differential environmental tolerances (Bemmels et al., 2018). In contrast, for some tree genera of rainforest trees, dispersal does not seem to be a constraining factor, meaning that community assemblages either represent random draws from the possible species pools available (Dexter et al., 2017), or functional differences arising from different habitat tolerances. The geographic distributions of many riverine and floodplain taxa are limited by river basin watersheds, and opportunities for dispersal include river capture events (Albert et al., 2017). Finally, it is not enough to know where particular species occur; we also need to know where these species do not occur (Soria-Auza & Kessler, 2008). It is, therefore, difficult to reliably say if the biodiversity patterns known to date really reflect true patterns or biases in collection effort. Further, patterns of species richness are usually discerned relatively early in the documentation of a newly explored biota, whereas patterns of species endemism are more difficult to discern as they require positive knowledge of both where species are present and absent (Soria-Auza & Kessler, 2008). |
Introducing “trans-Disciplinary Biogeography”
Here, we propose that the best way to fully understand the complexity of Neotropical biodiversity is by conceptualizing and implementing a novel holistic framework. We define trans-disciplinary biogeography as “a holistic framework that takes advantage of the methods and data in multiple disciplines, in order to solve complex questions about the evolution, maintenance, and distribution of biodiversity through time and scape. By doing so, each individual discipline transcends its traditional borders.”
The idea and need of combining data from different sources in biogeography has been advocated before (e.g., Ribas et al., 2012; Weeks, Claramunt & Cracraft, 2016) but we propose a major expansion. Some examples of the constituent disciplines in this pursuit include biology (e.g., community ecology, phylogeography, systematics, taxonomy, historical biogeography; Lomolino, Riddle & Whitakker, 2017), geology (e.g., palaeontology, sedimentology, geomorphology), and climatology (e.g., modeling, speleology), amongst others. Successful examples of trans-disciplinary research include the archaeology-ecology synergy that led to the elucidation of pre-Columbian effects on the distribution of Amazonian plants (Levis et al., 2017); the genetic-geology synergy that led to the discovery of an earlier and more prolonged biotic interchange between South and North America since the Miocene (Bacon et al., 2015; De Baets, Antonelli & Donoghue, 2016), and the geology-biogeography-systematics synergy that led the discovery of a Miocene origin for the modern transcontinental Amazon river (Hoorn et al., 2010b; Albert, Val & Hoorn, in press). Some of these interactions are already recognized as new sub-disciplines, such as “community phylogenetics” (Swenson, 2011), “geogenomics” (Baker et al., 2014), and “geodiversity” (Gray, 2004). We envision the integration of a high number of additional synergistic sub-disciplines.
In practice, we want to encourage young students and researchers to invest time in learning more about disciplines that might fall outside their general curriculum, but which could contribute to creating fruitful synergies. Obviously, not every project or publication has to (or should) be trans-disciplinary, and this pursuit should not decrease the depth of a researcher’s skills in her or his topic of expertise. But without trans-disciplinary frameworks that are defined in early stages of new research projects, there is a risk that important perspectives are missed out.
To showcase the benefit of these interactions, we provide some background on the emergence of Neotropical biogeography as a research focus—which was integrative from its early days, but successively lost much of its cross-disciplinarity. We then discuss how trans-disciplinary biogeography may help address the interactions between landscape evolution, climate change and biotic diversification at its multiple levels (see also Hoorn, Perrigo & Antonelli, 2018).
Early ideas about Neotropical biogeography
The Prussian naturalist Alexander von Humboldt was among the first to realize that biotic and abiotic processes interact to constrain species distributions, and to place these influences into a geological and climatic framework. He came to this notion in the Neotropics, most famously during his study of the Chimborazo volcano in Ecuador, where he carefully documented the location of different species along elevational zones (Humboldt & Bonpland, 1805). It was in this trip that he first observed that physical parameters such as topography and climate were key for geographic distributions.
A century later, Wegener (1912) advanced the incipient field of historical biogeography with the theory of continental drift, based in part on past geographic distributions of biotas linked by previously connected continental plates. The striking fit between the coastlines of South America and Africa was one of the pieces of evidence inspiring Wegener’s theory of dynamic, non-static landmasses. In the 1960s, a geophysical mechanism for plate tectonics was proposed (Vine & Matthews, 1963), placing studies of plant and freshwater fish biogeography into a plate tectonic framework, where vicariance was assumed as a major biogeographic force (Raven & Axelrod, 1974; Rosen, 1975).
At first, the explanatory power of vicariance biogeography was the ability to predict biogeographic distributions of individual taxa and that of whole biotas from knowledge of how landscapes changed through time (Rosen, 1978). The paradigmatic example is the geological fragmentation of the Gondwana supercontinent, and the resulting fragmentation of the resident Gondwanan biotas. The vicariance biogeography approach satisfies the scientific impulse of systematists and biogeographers for general explanations of organismal distributions, rather than ascribing each distribution to the vagaries of idiosyncratic evolutionary histories (Humphries & Parenti, 1999).
Soon after, the challenge to vicariance biogeography as a general theory was the commonplace observation that vicariant cladogenesis (i.e., allopatric speciation) is only one of three general macroevolutionary processes, along with dispersal and extinction (MacArthur & Wilson, 1967; Ree & Smith, 2008). Indeed, ecologists have long understood dispersal to be a pervasive process influencing biogeographic distributions (Cowie & Holland, 2006). Long-distance dispersal has been documented in the formation of many biotas worldwide (Sanmartín & Ronquist, 2004; Bacon, Baker & Simmons, 2012) including those in the Neotropics (Smith et al., 2014; Tagliacollo et al., 2015b; Hawlitschek, Ramírez Garrido & Glaw, 2017; Antonelli et al., 2018).
Inferring landscape evolution in the Neotropics
Neotropical historical biogeography increasingly relies on geological models that specify the landscape configurations on which species originate, disperse, and go extinct. Understanding phylogeny and biogeography in the context of landscape evolution requires assessment of geological data, including sedimentary environments, sedimentation rates, palaeontological records, and geochronological ages, among others (Salo et al., 1986; Räsänen et al., 1995; Räsänen, Salo & Kalliola, 1987; Hoorn et al., 1995; Lundberg et al., 2000; Figueiredo et al., 2009; Hoorn et al., 2010b; Lagomarsino et al., 2016; Sanín et al., 2016; Jaramillo et al., 2017; Hoorn et al., 2017).
Some recent reconstructions of the Neogene landscape in Amazonia are based on dynamic topography, in which mantle movements through time are quantified (Shephard et al., 2010). The effects of these movements are estimated on surface subsidence and are then related to environmental and landscape changes, such as the model applied to explain the genesis of the Pebas wetland in western Amazonia (Hoorn et al., 2010a). Another approach is to use numerical modeling and create reconstructions from physical parameters such as rates of erosion and mountain uplift. An example is the reconstruction of the flow of the Amazon River which incorporates surface processes, flexural isostasy and crustal thickening due to orogeny into a mathematical model to explain the drainage reversal in the Miocene (Sacek, 2014). However, this study did not incorporate the synergic effects of plate movements and surface dynamics, that are known to have impacted on the formation of mega-wetlands and ecosystems (Horton, 2018).
Landscape evolution models (LEMs) can be useful in a biological context but often lack spatial and temporal precision. Biological data can help to infer past landscapes, by testing alternative geological models and increasing their precision. However, we caution that the evolutionary history of one clade might represent an idiosyncratic story, rather than inform the general evolution of an entire landscape in which the clade occurs (Cruz-Neto, Garland & Abe, 2001; Ribas et al., 2012).
In recent years, integrated approaches have built integrative LEMs based on geological, climatic and biodiversity data (Craw et al., 2016; Badgley et al., 2017; Costa et al., 2017). Some studies make use of geographic information systems and combine these with well-dated palynological databases, such as Neotoma (https://www.neotomadb.org/). These models are mainly applied to reconstruct landscapes across Quaternary time scales (i.e., the past 2.6 million years). For example, reconstruction of changes in connectivity across the northern Andes enabled the inference of cyclic phases of biotic dispersal and speciation vs. extinction (Flantua et al., 2015). Molecular phylogenetic data can be used to statistically evaluate the likelihood of competing geological models on longer time scales, such as the closure of the Central American Seaway dividing South and Central America (Bacon, 2013), and the roles of the Caribbean plate margins as dispersal corridors between South and Central America (Tagliacollo et al., 2015a). Similar approaches based on both terrestrial (Baker & Couvreur, 2012) and aquatic taxa (Hrbek, Seckinger & Meyer, 2007) may provide important insights when geological data are insufficient or ambiguous.
The impact of the Andean uplift on Neotropical diversification
Neotropical biodiversity can only be properly understood when considering the Andean uplift and the effects of this orogeny on the landscape (Fig. 3) and regional climate (Räsänen, Salo & Kalliola, 1987; Gentry, 1982; Hoorn et al., 2010b). Although the Andes are entirely confined to South America, their formation has led to far-reaching effects across the Neotropics, and there are clear links with orogenies in Mesoamerica caused by plate tectonics.
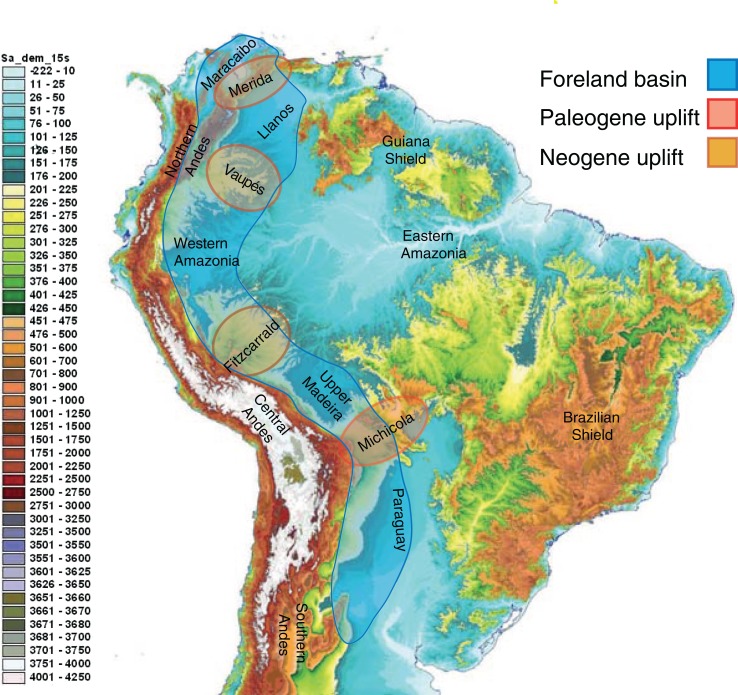
Figure 3. The complex topography and geology of South America.
This map highlights the topographic differences across the continent, including the Precambrian and Paleozoic upland shields, and the Andean cordilleras and structural arches that uplifted during the Cretaceous and Cenozoic. The Sub-Andean foreland basin constituted the main drainage axis of South America for most of the past 100 million years, serving as the main arena of evolutionary diversification for the mega-diverse biota of lowland Amazonia. Uplift of structural arches during the Paleogene and Neogene resulted in the formation of the modern continental drainage configuration. Base map created by Paulo Petry from the Shuttle Radar Topography Mission with elevations in meters. Note that the scale exaggerates differences at lower elevations. Adapted from Albert, Petry & Reis (2011).
The Andes today
The 7,000 km long Andes is positioned perpendicular to the principal global atmospheric currents and traps the humid air of the Intertropical Convergence Zone. This configuration enhances precipitation along the Andean slopes and in western Amazonia, making them wetter than they would be in a low Andes setting. Moreover, the high Andes also redirects the atmospheric flow inducing the southward deflected South American low-level jet (Garreaud & Muñoz, 2005; Insel, Poulsen & Ehlers, 2010; Rohrmann et al., 2016).
The situation is reversed in southern and in northwestern South America. In these regions, the Andes trap the humid air of the Southern Hemisphere westerlies (Garreaud & Muñoz, 2005). In contrast to the Amazonian settings, the eastern margin of the Andes at its northern and southern extremes forms a rain shadow where semi-desert conditions prevail, and on the western flank there is increased precipitation with more humid conditions (Blisniuk et al., 2005; Palazzesi et al., 2014), although this situation is partially reverse during El Niño events. The monumental Andean barrier has thus imposed strong impacts on both the climate and landscapes of South American lowlands, resulting in the modification of river systems and drastic changes in the climate and habitats of many Neotropical regions.
Impact on biotic diversification
The rise of the northern Andes had a major impact on Neotropical biodiversity, as documented for many taxa (Cruz-Neto, Garland & Abe, 2001; Hughes & Eastwood, 2006; Santos et al., 2009; Tagliacollo et al., 2015b; Sanín et al., 2016; Chazot et al., 2016; Diazgranados & Barber, 2017; Bacon et al., 2018). Recent studies that explicitly integrate surface uplift and climatic changes as a function of speciation and extinction include work on the Andean bellflowers (Lagomarsino et al., 2016), Neotropical orchids (Pérez-Escobar et al., 2017), and Neotropical hummingbirds (Condamine et al., 2018).
The Andean uplift affected Neotropical regions in different ways. Over the course of the Miocene, it led to a humidification of Amazonia and aridification of Patagonia (Cione et al., 2005; Blisniuk et al., 2005; Palazzesi et al., 2014; Rohrmann et al., 2016). This contrast is reflected by the history of New World monkeys (Platyrrhini), whose geographic expansion and morphological and taxonomic diversification is tightly linked with climatic changes (Silvestro et al., in press). Platyrrhines were once widely distributed in Patagonia from early to middle Miocene, including the southernmost non-human primates that have ever lived (Tejedor et al., 2006; Novo et al., 2017). However, those primates were later extirpated during regional aridification and global cooling after the Middle Miocene. The platyrrhine record of the high Chilean Andes indicates that the connection between Patagonia and the northern Neotropics possibly persisted on the western part of South America, as the southern Andean cordillera was not an important barrier in the Middle Miocene (Flynn et al., 1995). This scenario provided primates and other animals with a migration route to the north, facilitating faunal turnover (Tejedor & Muñoz-Saba, 2013). This connection may also have contributed to the subsequent Amazonian diversification of crown platyrrhines, including some Patagonian lineages (Rosenberger et al., 2009).
Advances on climatic reconstructions via historical records and climatic modeling (Cheng et al., 2013; Wang et al., 2017) or biome palaeo-distribution modeling (Carnaval & Moritz, 2008; Werneck et al., 2011, 2012a; Ledo & Colli, 2017) allow for direct hypothesis testing based on independent biodiversity data.
The Four Scales of Biodiversity Research
In the previous section, we urged for a broader integration across the scientific disciplines. We exemplified “trans-disciplinary biogeography” by showcasing the strong links that exist, for instance, between geological and biological fields. Now, we wish to deepen the discussion within biodiversity research in a more traditional sense. We do this by discussing and contrasting the current and potential levels of interaction across four sub-disciplines that span the taxonomic, temporal and spatial scales: (1) community ecology, (2) phylogeography, (3) phylogenetics, and (4) historical biogeography (Fig. 4).
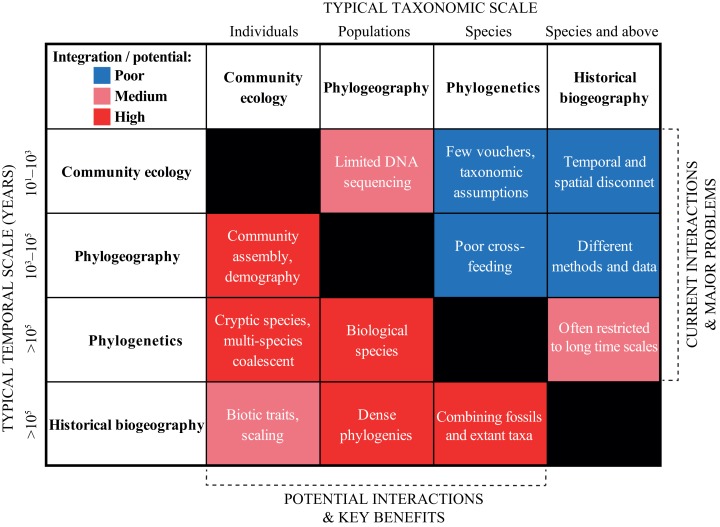
Figure 4. Heat map summarizing the current (right upper boxes) and potential (left bottom boxes) interactions across the biodiversity disciplines in the Neotropics.
The X- and Y-axes indicate the typical taxonomic and temporal scales covered by each discipline, respectively. The white text in each box provides some short examples of why the disciplines are not yet successfully integrated, and some of the key benefits that will be gained by a further integration. See text for a discussion.
Community Ecology
A major question in the study of biodiversity in general, and Neotropical research in particular, is how ecological communities have been assembled over time and how abiotic factors and species interactions have influenced this process. Approaches for the study of biodiverse communities have employed a wide range of models with diverse conceptual roots. Over the last 20 years, there has been an expansion from studies focusing on contemporary community structure and spatial patterns of physical properties of ecosystems (Tuomisto, Ruokolainen & Yli-Halla, 2003; Heithaus, 1979; Gentry, 1988; Duellman, 1989; Tuomisto et al., 1995; Kalko & Handley, 2001; Garnier et al., 2004), to studies focusing on historical aspects of community structure and evolution (Leite & Rogers, 2013; Smith et al., 2017). Early approaches (Margalef, 1963) focused on indices of diversity, descriptions of community membership, as well as flow charts of energy and nutrients through the community. Key variables influencing community structure consistently emphasized classic Hutchinsonian processes such as resource use, competition, and niche partitioning. However, as ecologists adopted new techniques, the resolution of the niche increased from simple variables to also include high-resolution data on climate, soil chemistry, microbiomes, and other physical and biological properties. These approaches have guided several recent analyses of tropical groups, including microorganisms and plants (Tuomisto et al., 2003; Costa et al., 2009a; Mendes et al., 2015; Arellano et al., 2016; Tuomisto et al., 2016). Additionally, the availability of spatially explicit online global datasets of climate and environmental parameters has helped spawn a generation of studies using large-scale spatial biodiversity surveys and inventories. When analyzed with statistical approaches, these data allow the interpolation between sampled sites and estimation of diversity in non-sampled areas (Costa et al., 2007, 2009b; Ter Steege et al., 2011, 2013).
Niche-based studies
The general idea that species are adapted to their environment (i.e., have different niches) has two important consequences. First, species distributions are expected to reflect the distribution of suitable habitats. Second, species composition in local communities should reflect the environmental characteristics of the site, as unsuitable environmental characteristics or biotic interactions make it impossible for a species to establish and/or survive. Along these lines, many studies have aimed to characterize the edaphic associations of tropical plant species (Tuomisto & Poulsen, 1996; Phillips et al., 2003; Costa, Magnusson & Luizao, 2005; Roncal, 2006; Zuquim et al., 2009; Kristiansen et al., 2012; Tuomisto et al., 2016; Cámara-Leret et al., 2017; Figueiredo et al., 2017) and the elevational ranges of many taxa (Kluge, Bach & Kessler, 2008). If there are more species adapted to some environmental conditions than others and dispersal is generally not a limiting factor, a species richness gradient should result. However, it is also possible that some environmental conditions may allow more species to coexist than others. Several studies have analyzed species richness gradients along environmental gradients such as elevation (Kluge, Kessler & Dunn, 2006; Brehm, Colwell & Kluge, 2007), rainfall (Clinebell et al., 1995; Esquivel-Muelbert et al., 2017), and soil fertility (Costa, Magnusson & Luizao, 2005; Ter Steege et al., 2006; Tuomisto, Zuquim & Cárdenas, 2014). In general, these studies have shown that Neotropical plant species richness tends to be highest in warm, humid, and aseasonal environmental niches at low to middle elevations.
Neutral and non-neutral perspectives
In contrast to niche-based processes, spatial patterns in the abundance of anurans from Central Amazonia have been shown to conform to the expectations of Hubbell’s neutral theory of biodiversity and biogeography (NTBB; Hubbell, 2001; Diniz-Filho et al., 2011). More recently, a study demonstrated that the incorporation of population genetic dynamics into NTBB support the hypothesis that biodiversity dynamics are out of equilibrium (Manceau, Lambert & Morlon, 2015). Additional research is needed to assess the relative roles of niche constraints, neutral, and non-neutral processes, in explaining and predicting Neotropical biodiversity.
Ecological interactions
Early theoretical ecologists conceived the role of ecological interactions (e.g., hervivory, pollination, frugivory) in shaping natural communities, mainly through mechanisms of competition and predation (Hazen, 1964; Boucher, 1988). Later, theoretical ecology shifted to a broader perspective, when facilitation (i.e., positive interactions such as mutualism and symbiosis) was envisioned as a mechanism that affects processes in both population and community levels (Bruno, Stachowicz & Bertness, 2003). This broad spectrum linked many ecological concepts and type of data (such as geo-referenced occurrences and DNA sequences), creating a multi-layer framework for investigating macro-evolutionary processes and patterns.
The use of multi-layered data have shed light on the role of climate gradients in pollinator turnover (Correa Restrepo et al., 2016), the role of frugivory traits in palm diversification (Onstein et al., 2017) and demographic and spatio-temporal distribution of species interactions (Beck, 2006). Coupling time-calibrated phylogenetic and ecological data of ant-plant interactions in the Neotropics also allowed the reconstruction of the geographical origin of the Acacia-ant interaction (Gómez-Acevedo et al., 2010) and the identification of ecological and macro-evolutionary patterns in ant symbioses (Chomicki, Ward & Renner, 2015). In addition, phylogenetic and network analyses disclosed that specialized pollination interactions can display asymmetrically dependent diversification (Ramírez et al., 2011), revealing that specialized interactions might dilute the ecological signal in macro-evolutionary processes. Furthermore, comparative phylogenetic analyses using multi-layered data suggest that mutualistic interactions drive the relative higher diversification rates of frugivorous bats in some Neotropical regions (Rojas et al., 2012) and highlight the putative role of bat seed dispersal in shaping species-rich meta-communities.
Phylogeography
Phylogeographic research based on dense geographic and molecular sampling at the intraspecific level (subspecies and populations) has advanced significantly in the Neotropics. Specifically, phylogeographic studies of widespread species has improved our understanding of genetic diversification across various biomes. It might be intuitive to assume that species are confined to particular biomes, so that most phylogeographic studies should be done at such spatial scale, but this might not necessarily be the case. We addressed this possibility by synthesizing the recent data presented in Antonelli et al. (2018), where all available Neotropical species in six major clades were codified as present or absent in ten broadly defined biomes or regions: Andean Grasslands, Amazonia, Atlantic Forests, Caatinga, Cerrado and Chaco, Dry Northern South America, Dry Western South America, Mesoamerica, Patagonian Steppe, and West Indies. Our compilation shows that a substantial proportion of species in each clade occurred in more than a single biome or region: angiosperms (27,875 species or 36% of the total number of species analyzed), frogs (232 spp., 17%), birds (1,440 spp., 43%), ferns (1,529 spp., 40%), mammals (530 spp., 42%), and squamates (482 spp., 23%). Clearly, cross-biome transitions have taken place at various taxonomic levels, including populations and species.
Examples of phylogeographic studies across biomes include investigations of major vegetation transitions between Amazonia and the Cerrado (Gehara et al., 2014; Melo et al., 2018) and across biomes with closer functional affinities, such as those of the dry diagonal (Werneck et al., 2012a). Another recent study revealed phylogeographic patterns of disjunctly distributed taxa, which led to inferences on the past connectivity among biomes (Thomé et al., 2016; Prates et al., 2016b, 2016a). It should be noted, however, that at least some of the species of apparently widespread distribution across multiple biomes may in fact not have left their preferred habitat—such as Amazonian species being found along gallery forests in the Cerrado (see Discussion in Antonelli et al., 2018).
Comparative phylogeographic approaches can lead to robust inferences of lineage diversification, and even challenge traditional allopatric scenarios, as has been shown for Neotropical rainforest birds (Smith et al., 2014), or for the synchronous demographic expansion detected for the xeric Caatinga herpetofauna (Gehara et al., 2017). In the Neotropics, hierarchical approximate Bayesian computation analyses (Hickerson, Stahl & Lessios, 2006) have been a popular option to reconstruct patterns of shared phylogeographic history (Carnaval et al., 2009; Prates et al., 2016a; Gehara et al., 2017). More recently, a paradigm shift has been proposed for the field of phylogeography, arguing for a focus placed on trait-based hypothesis testing rather than the more traditional approach of concordance amongst taxa (Papadopoulou & Knowles, 2016). Although this is a relatively new advance in the field, it may gain popularity, considering the general lack of concordance in the distribution of many taxa and the possible range of underlying causes. For instance, a recent study of Neotropical fishes showed that population structure is not concordant amongst palaeodrainages, but rather reflects the fundamental differences in riverine history (Thomaz, Malabarba & Knowles, 2017).
Phylogenetics
The explosion of molecular phylogenetics and dating analyses allow the inference of time-calibrated trees, where branch lengths are measured as units of time or rates of molecular evolution. Fortunately, a massive increase in the availability of genetic information is being driven by high throughput sequencing technologies. Novel genomic data are likely to significantly improve our understanding of genetic diversity and evolutionary relationships among and within species (Jin & Chakraborty, 1994). Furthermore, these data will also greatly improve our understanding of largely under-studied groups, such as soil microbes (Mahé et al., 2017).
The integration of time-calibrated trees into phylogeographic and biogeographic analyses now enables the establishment of links with external sources of temporal information such as landscape evolution, geological history, fossil record, and climate history. Therefore, phylogenies constitute the strongest and most concrete “bridge” across scales and disciplines as outlined here (Fig. 4). Given the complexity and challenges with the reconstruction of reliable phylogenetic trees, we refer to an accompanying review in this volume (Bravo et al., 2018).
Historical Biogeography
Single clade approaches
Detailed reconstructions of the temporal and spatial evolution for individual clades are obtained through “single clade” approaches. These approaches focus on contingencies or events that are idiosyncratic to the group under study, instead of generalities across groups. Methodological advances in single lineage approaches have undergone major developments with parametric methodologies (Ree & Smith, 2008; Lemey et al., 2009; Landis et al., 2013; Matzke, 2014; Landis, 2017; Table 7).
Table 7. Methodological challenges and advances for estimating biogeographic histories.
| Inferring the spatial and temporal dimensions of evolution are fraught with difficulties, especially due to a lack of abundant and evenly sampled biological and geological data. This is particularly critical for the Neotropics, due to the region’s immense size, relatively limited access, extraordinary biodiversity levels, landscape heterogeneity, and complex evolutionary and geo-climatic histories. To tackle these problems, we summarize some of the main issues associated with the analyses of biogeography and diversification, focusing on how those issues affect the inference of geographic range evolution of lineages in the Neotropics. |
| Definition and use of areas for analyses. Defining units of study in biogeography is not an easy task, especially when diverse systems are involved such as the Neotropics. Sympatry, or the geographic congruence among the distribution areas of taxa, is often used as a criterion to define units for these studies. The identification of such areas has long been based on expert opinion, with data-driven approaches that use actual species distribution data only becoming available more recently (Holt et al., 2013; Vilhena & Antonelli, 2015; Edler et al., 2016; Antonelli, 2017a, 2017b). These approaches to bioregionalization allow for more objective and reproducible analyses. Areas have also been defined using geologically explicit criteria, including information on the geological history of landmasses or geographic barriers, both of which are not exclusive to the group under study (Antonelli et al., 2009; Albert et al., 2011; Töpel et al., 2016; Bacon et al., 2018). Areas defined based on species distribution patterns and geological history are of particular interest (Perret et al., 2007; Givnish et al., 2014; Tagliacollo et al., 2015b). |
| The use of areas as discrete entities is useful in parametric biogeographic models where areas are considered as traits that evolve along the phylogeny, and whose ancestral areas are estimated at speciation events (nodes). In these models, the spatial units of analysis are defined by the biogeographic hypothesis under examination. For example, it is possible to determine whether diversification rates have been historically higher in Andean or non-Andean taxa (Chazot et al., 2016). However, defining areas as discrete entities is difficult when there are overlapping boundaries and an excess of widespread taxa. Models have been proposed to objectively define areas of endemism by overlapping taxa with “fuzzy” boundaries (Szumik et al., 2002; Szumik & Goloboff, 2004). Similarly, biotic element analyses have also been proposed to test for non-random distributions of species ranges (Hausdorf & Hennig, 2003). Some of these methods have been applied to Neotropical taxa (Casagranda, Roig-Juñet & Szumik, 2009; Guedes, Sawaya & Nogueira, 2014; Noguera-Urbano & Escalante, 2015; Azevedo, Valdujo & Nogueira, 2016). |
| Alternatives to the use of areas. One possibility is to use geographic barriers, rather than areas, as units of analysis, thus focusing on vicariance (Hovenkamp, 1997; Arias, Szumik & Goloboff, 2011; Arias, 2017). This approach explicitly introduces the spatial (landscape) aspect missing from the predefined areas-as-discrete entities used in parametric biogeography. Since this approach is based on taxon-defined ranges, biogeographic reconstructions are not dependent on different area definitions (Arias, 2017). A parametric version of this approach allows geographic (dispersal) barriers to evolve over time within the landscape, something that has been particularly useful for understanding the biogeographic history and evolution of Neotropical freshwater fishes (Albert et al., 2017). |
| An additional alternative to using discrete areas in biogeographical analyses is the spatial diffusion approach, which conducts spatial-temporal reconstructions under random walk models within likelihood (Lemmon & Lemmon, 2008) or Bayesian (Lemey et al., 2010) frameworks. This approach has been used to study taxa from dry Neotropical biomes (Werneck et al., 2011, 2012a; Nascimento et al., 2013; Camargo et al., 2013), and taxa with broad continental distributions (Gehara et al., 2014). A further development of this approach has been applied to the Neotropical bird genera Psophia and Cinclodes (Quintero et al., 2015). The method uses georeferenced point-localities to infer ancestral areas and thus does not make assumptions about species ranges and operational units that fit many taxa. On the other hand, this method suffers from the common issue of ancestral lineages occupying average values of the descendant lineages. For instance, analyses with this method have reconstructed the ancestral of Cinclodes ovenbirds to a region in-between the western and eastern margins of South America, where no such species occur today (Quintero et al., 2015). Considering the complex and dynamic nature of the Neotropical region, diffusion analyses would certainly benefit from the incorporation of landscape-explicit models that allow the reconstruction of actual paths along branches and fossil-informed diffusion approaches (McRae et al., 2008; Meseguer & Condamine, 2018). These developments would allow the incorporation of spatial heterogeneity via dispersal constraints, derived from estimated ecological niche models or landscape evolution models. |
| Estimating geographic evolution on single clades. Dispersal-extinction-cladogenesis (DEC) is likely the most popular parametric biogeographic method for estimating the geographic evolution of lineages within a particular clade. This likelihood-based method infers anagenetic evolution (i.e., along branch internodes) as a function of two rate parameters: range expansion (dispersal) and range contraction (local extinction). Cladogenetic evolution (i.e., at speciation nodes) is modeled as the likelihood of alternative range inheritance scenarios that describe the division of ancestral ranges into descendant nodes: sympatric speciation or allopatric (vicariance) speciation, and peripheral isolate speciation in the case of widespread ranges (Ree & Smith, 2008). |
| The popularity of DEC is based on the fact that, given a time tree and associated terminal distributions, it can provide detailed biogeographic reconstructions of the ancestral origin of a clade and the history of dispersal and extinction events that shaped its spatial evolution (Sanmartín & Meseguer, 2016). A potential drawback of DEC is, however, the number of areas that it can implement. A large number of unit areas rapidly leads to computational and convergence issues. Constraining the number of states based on biological or geological criteria is a way to decrease model complexity (Ree & Sanmartín, 2009). |
| Bay-area, a data augmentation approach based on stochastic mapping that extends the DEC model to deal with a large number of unit areas, has been proposed to tackle the limited number of areas allowed in DEC (Landis et al., 2013). Another extension of DEC is the DEC+J model, which introduces an extra parameter (“J”) to model “jump dispersal” or “founder-event” speciation (Matzke, 2014). The DEC+J model has been used in Neotropical biogeography (Matos-Maraví et al., 2014; Chomicki & Renner, 2015; Espeland et al., 2015), but was recently criticized due to statistical bias (Ree & Sanmartín, 2018). Recently, a new extension was introduced to allow for a time-heterogeneous dispersal process in a Bayesian framework, the “epoch model” (Landis, 2017). This model can be used in the biogeographic dating of speciation events when no fossil or other calibration method exists, and was recently applied to Neotropical cycads (Said Gutiérrez-Ortega et al., 2017). |
Typical biogeographic analyses use time-trees and parametric models of biogeographic evolution to estimate ancestral ranges of lineages (branches) and speciation events (nodes), and to infer rates of biogeographic processes (e.g., dispersal, speciation, and extinction). To date, probably hundreds of studies have examined the biogeographic history of particular Neotropical clades in this way. Biogeographic hypotheses or models about the relative role of biogeographic processes in the geographic evolution of particular groups can be compared statistically using methods for model selection in phylogenetics, such as the Akaike information criterion or Bayes factors (Bozdogan, 1987). Moreover, the rates of these processes may be modified (scaled) to reflect the changing connectivity among the areas of analysis over time (Ree & Smith, 2008). These advances have contributed to the integration of landscape dynamics and geological history into taxon biogeography in the Neotropics (Givnish et al., 2014; Palazzesi et al., 2014; Tagliacollo et al., 2015b; Chazot et al., 2016).
Cross-taxonomic (multi-clade) approaches
These approaches (sometimes under the umbrella of “comparative biogeography”; Antonelli, 2017b) aim to extract generalities on the evolution of a biogeographic region or whole biota, or generalities on the relationships among biogeographic regions or biotas, by reconstructing the history of their individual components. The focus of this approach is on inferring shared biogeographic histories, such as general patterns of colonization and diversification or a common response to climatic and landscape changes. A recent cross-taxonomic analysis for six major clades of terrestrial plants and animals, across all major Neotropical biomes and regions, was presented by Antonelli et al. (2018). That study showed an unexpectedly high number of dispersal events across the entire Neotropics, which took place for tens of millions of years and often involved shifts in major environmental types (in particular from forests to savannas). The high frequency of dispersal events identified in that study reflects patterns reported for tree communities across Neotropical rain forests (Dexter et al., 2017) and between rainforests and savannas (Simon et al., 2009).
Multi-clade approaches were traditionally known as “area biogeography” and were the focus of the cladistic biogeographic school for decades (Nelson & Platnick, 1980; Humphries & Parenti, 1999). The first methods used for cross-taxonomic biogeographic approaches were based on parsimony, which does not allow the formal integration of a temporal dimension (Crisci et al., 1991; Marshall & Liebherr, 2000; Sanmartín, 2016). The incorporation of time into event-based methods then allowed the identification of dispersal corridors and barriers, such as those underlying the assembly of freshwater fish faunas in South American river basins (Dagosta & De Pinna, 2017). Recently developed parametric approaches (Ronquist & Sanmartín, 2011; Sanmartín, 2016) offer now a powerful way to obtain generalities about patterns of dispersal and diversification in biotas, allowing us to test between alternative geological or spatial scenarios (Sanmartín, Van Der Mark & Ronquist, 2008). An interesting methodology bridging community ecology and cross-taxonomic biogeographic analysis is the phylogeographic concordance factor analysis (Satler, Zellmer & Carstens, 2016), which uses Bayesian concordance analysis (Ané et al., 2007) to test for shared evolutionary history among co-distributed species and the existence of strong ecological interactions or dependence (Satler & Carstens, 2016, 2017).
Bridging the Classical Biodiversity Disciplines
Previous sections described the complexity of Neotropical biodiversity, outlined major knowns and unknowns, proposed a general integrative framework, and discussed approaches and applications of methods depending on the scale. Here, we provide a few examples of how to expand beyond the traditional boundaries and scales of the disciplines related to those in Fig. 4.
Assembling biodiversity: from communities to biotas
The Theory of Island Biogeography (TIB; MacArthur & Wilson, 1967) introduced parameters such as rates of colonization (immigration) and extinction within a mathematical framework, allowing the prediction of the number of species present on an island based solely on its distance from a mainland species source and its area (Losos & Ricklefs, 2009; Warren et al., 2015). New models inspired by the TIB are now attempting to integrate additional parameters, such as speciation and island age (Whittaker, Triantis & Ladle, 2008), population abundances (Rosindell & Harmon, 2013), and trophic interactions (Gravel et al., 2011).
Community ecology and an expanded TIB are now also adopting a more evolutionary approach by integrating phylogenetic data to the study of community assembly and dynamics, including the role of in situ adaptation or speciation vs. dispersal in community assembly, the temporal sequence of species interactions, and the role of abiotic and biotic factors in diversification of specific lineages (Webb et al., 2003; Sanmartín, Van Der Mark & Ronquist, 2008; Kursar et al., 2009; Valente, Etienne & Phillimore, 2014; Valente, Phillimore & Etienne, 2015, 2018; Cabral, Valente & Hartig, 2017). Importantly, this requires denser voucher sampling of specimens, which in turn will lead to denser phylogenies, better estimates of species boundaries under multi-species coalescent approaches and tackling the common problem of cryptic species (Fig. 4; Bravo et al., 2018). By adopting a more historical focus, community ecology methods are explicitly trying to reconstruct the sequence of events leading to modern-day communities, such as the island-like patches of white-sand savannas in Amazonia (Alonso, Metz & Fine 2013). These approaches relax the assumption of ecological neutrality, and focus on the distinctive properties of individual lineages, historical contingency, and particularities of present-day outcomes (Emerson & Kolm, 2005; Sanmartín, Van Der Mark & Ronquist, 2008). In their most recent forms, these models incorporate ecological parameters such as competition and species interactions (Clarke, Thomas & Freckleton, 2016) or landscape dynamics (Aguilée, Claessen & Lambert, 2013).
Scaling up community ecology approaches
The original goals of community ecology, as established in the early 20th century, were to predict species distributions and abundances, species richness and equitability, community productivity, food web structure, predator-prey dynamics, succession, and community assembly. However, this discipline has not yet succeeded in meeting most of these goals (Ricklefs, 2008; Ritchie, 2009; Vellend, 2010; Ricklefs & Jenkins, 2011; Weber & Strauss, 2016). The reasons are many, but may be especially associated to the non-equilibrium condition of most local assemblages, in which the effects of historical contingencies of dispersal, extirpations, and other stochastic processes override the equilibrium expectations generated by local functional processes such as predation and competition (Fig. 5). In other words, the species composition and equitability of most local assemblages are more strongly governed by regional and historical factors than by local ecological interactions (Mittelbach & Schemske, 2015; Manceau, Lambert & Morlon, 2015; Fukami, 2015; Weeks, Claramunt & Cracraft, 2016).
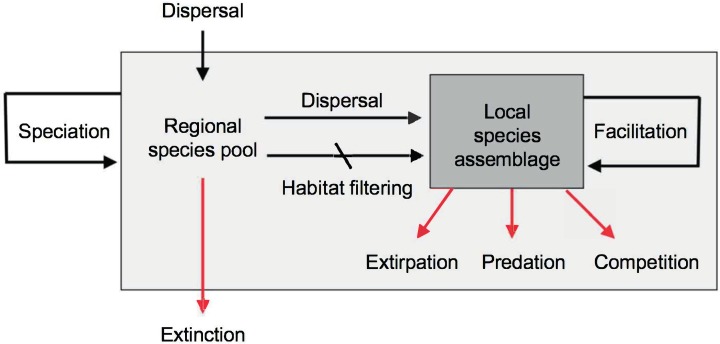
Figure 5. Main evolutionary and ecological processes contributing to the formation of species richness.
The regional species pool (light gray box) is defined as the sum of all the local species assemblages (darker gray box). Black arrows indicate processes that increase species richness, red arrows processes that reduce species richness. Note the hierarchical organization of processes resulting in species richness, with evolutionary processes occurring over regional to continental spatiotemporal scales and ecological processes occurring over local scales. Speciation and dispersal contribute new species to the regional pool, while extinction removes species. Dispersal mediated by abiotic habitat filtering and biotic facilitation (Kraft & Ackerly, 2014) increase the richness of local assemblages by enhancing establishment of species preadapted to local conditions, or aiding in the establishment of other species. Biotic interactions such as predation and competition may serve to reduce local richness. Diagram modified from Schluter & Ricklefs (1993) and Albert, Val & Hoorn (in press).
This “crisis” in community ecology has fueled the rise of alternative functionally-neutral theories, like the NTBB (Hubbell, 2001), and the metacommunity theory (Leibold et al., 2004). However, neutral theories have also been criticized for their simplistic assumptions and lack of predictive power under the non-neutral conditions frequently observed in nature (McGill et al., 2006). In general, the field of community ecology appears to be ripe for a paradigm shift (DeAngelis & Grimm, 2017).
While many studies conducted at continental to global scales aim to test broad hypotheses about drivers of biodiversity gradients (Tuomisto, Zuquim & Cárdenas, 2014; Fine, 2015), others rely on analyses of region-wide field data collected over decades (Ter Steege et al., 2013). These surveys set the stage for analyses on the environmental and historical correlates of diversity (Benavides et al., 2005; Stropp, Ter Steege & Malhi, 2009; Ter Steege et al., 2013). Detailed explanations of the heterogeneity found at multiple scales remain a major challenge.
One recent topic of concern is whether Neotropical biodiversity patterns documented today have resulted from purely “natural” processes, or have been largely influenced by human activities (Levis et al., 2017). Evidence from archaeological, remote sensing, biodiversity data, and modeling approaches suggest that humans may have had a much deeper impact on Neotropical biodiversity, both in time and space, than traditionally conceived (Table 8).
Table 8. Human impacts on Neotropical biodiversity.
| Humans have occupied the Neotropics since about the end of the Late Pleistocene (10–20 kya) and were likely instrumental in promoting the extinction of the diverse fauna of large-bodied mammals (Sandom et al., 2014). The drastic decrease in the density and diversity of large mammals also resulted in major changes to the overall vegetation structure (Bond, 2005). For example, in South America, the limits between the dry diagonal and the adjacent forests might have shifted significantly compared to where they would have been without any human involvement and its cascading effects (Doughty, Faurby & Svenning, 2016). In addition to anthropogenic extinctions, humans might also have caused drastic range contractions of many other species, and reduced the abundances of others (Faurby & Svenning, 2015). The human-linked reduction of the Neotropical megafauna may also have affected the plants that these animals dispersed. This pattern was recently discussed in the context of the impact of over-hunting of primates and tapirs on the total woody biomass of Amazonia (Peres et al., 2016), and large frugivorous mammals in the Atlantic forest (Bello et al., 2015). Overall, it seems that the patterns observed could reflect the pressures of overhunting in pre-historic times (before 500 years ago). Humans have restricted the ranges of some species, but actively or passively expanded the ranges of others, such as invasives or domesticated species (Levis et al., 2017, 2018). Knowledge to date is based on the best-studied groups and it remains unclear whether substantial effects of humans might be frequent among other organisms. We anticipate that this will become an active area of research for the coming years. |
| Apart from the effects of past human activity on Neotropical biodiversity, current habitat loss, climate change and neglected conservation strategies pose increasingly serious threats to natural landscapes. Indeed, these are widely known to be the primary drivers of the current global biodiversity crisis. Studies that quantify genetic diversity, vulnerability, and extinction risk derived from the impact of habitat loss and climate change are essential to grasp how current human activities are expected to impact the future of Neotropical diversity at multiple levels. Although we now have a fair understanding of several components of Neotropical biodiversity, for many taxonomic groups, well-defined processes remain elusive and biases loom large; refining these issues will constitute an area of active scientific exploration for the next decade and beyond. |
Exploring the tripod: ecological interactions-macroevolution-biogeography
The multi-layer analytical framework developed by recent eco-genetic research granted the combination of multiple data layers from three different scale dynamics (local mechanisms, macro-evolutionary processes, geographic patterns). The synergy of those layers illustrates a tripod that gathers ecological, evolutionary and biogeographical factors of populations, communities, and meta-communities, respectively (Ricklefs & Jenkins, 2011; Hanson et al., 2012; Connolly et al., 2017). Detecting ecological signals across multi-layered data, such as the contribution of mutualisms in biogeographic processes (speciation, extinction, migration) remains a major challenge. Tackling this challenge will require linking spatio-temporal data with models that detect common signatures of ecological interactions across layers (Pilosof et al., 2017). Although recent theoretical advances have unveiled phylogenetic signals from community processes (Rezende, Jordano & Bascompte, 2007; Minoarivelo et al., 2014; Bastazini, 2017), we urge for new models that can identify ecological signal from multiple layers. The exploration of ecological factors that are associated to positive and negative interactions (i.e., network structure, taxonomic associations) might reveal important insights on the dynamics and complexity of ecological interactions for producing and maintaining Neotropical biodiversity.
Incorporating fossils into biogeography
One important shortcoming of molecular-based biogeographical analyses in general, and parametric models of range evolution in particular, is the fact that it is almost always based on extant data alone. Because of the effects of extinction, the pattern of geographic distribution we observe today may be a poor representation of the actual biogeographic history, especially if extinction rates have been unequal among areas (Sanmartín & Meseguer, 2016) and taxa (Silvestro et al., 2016). One way to solve this issue is to include extinct lineages in biogeographic analyses (Mao et al., 2012), or to use their past distribution inferred from the fossil record to constrain inferences of ancestral ranges (Meseguer et al., 2015). This approach has often revealed different biogeographic histories for the study group as compared to analyses based on extant data only (Mao et al., 2012; Meseguer et al., 2015). A recent development is the development of the dispersal-extinction-sampling model, to infer rates of dispersal and area extinction exclusively from fossil data (Silvestro et al., 2014, 2015). Under this approach, a separate sampling parameter is used to account for the unevenness of the fossil record both spatially and temporally. When the fossil record is sufficiently abundant, it provides more accurate measures of changes in rates of geographic evolution and less biased extinction rates, than when exclusively extant taxa are used (Silvestro et al., 2015).
Another challenge to understanding current patterns of evolutionary diversity is the absolute dating of phylogenies, which relies heavily on fossils. This shortcoming complicates a detailed understanding of the ages of tropical taxa, especially those from rainforests (Wing et al., 2009). New methodological developments to directly integrate fossil (extinct) lineages into phylogeny reconstruction (Ronquist et al., 2012; Heath, Huelsenbeck & Stadler, 2014; Zhang et al., 2016; Silvestro et al., 2016) offer new hope in the quest to retrieve more accurate depictions of evolutionary patterns.
Finally, estimating the tempo of diversification is difficult without fossil constraints. In simulated phylogenies, the resulting shape of lineage-through-time plots vary significantly when the fossil record is added as compared to phylogenies that incorporate extant taxa exclusively (Matos-Maraví, 2016; Sanmartín & Meseguer, 2016). The inferred macroevolutionary dynamics estimated from molecular phylogenies may thus be misleading if fossil taxa are neglected, or when macroevolutionary tools do not acknowledge the rare sampling of fossil lineages. Clearly, fossils are crucial to not only understand past dynamics, but also for an improved understanding of current patterns (Fritz et al., 2013). We therefore urge for a much tighter integration between the palaeontological and neontological research communities in the Neotropics.
Integrating landscape evolution models into biotic diversification
A potential problem with single clade and cross-taxonomic biogeographic analyses as discussed above is that areas are treated as traits of organisms evolving along phylogenetic trees. Geology is often used to inform the model but does not form its core. For instance, area connectivity is often used in parametric methods to constrain or scale migration rates but not as an actual part of the model. A new generation of methods that use the power of LEMs to study the full panoply of evolutionary processes, at both microevolutionary (population) level (Byrne & Hopper, 2008; McRae et al., 2008) and macroevolutionary (interspecific) scales (Tagliacollo et al., 2015b; Badgley et al., 2017) are now being developed. For example, uplift of a dissected landscape and river capture are two landscape evolution processes with great power to generate high species richness (Albert et al., 2018). Both of these processes simultaneously and continuously merge and separate portions of adjacent landscape areas, allowing biotic dispersal and larger geographic ranges, vicariant speciation and smaller geographic ranges, and extinction when range sizes are subdivided below a minimum persistence threshold (Albert et al., 2017).
Conclusions
The origins of Neotropical biodiversity
There are often mixed definitions and questions related to the timing and mode of biotic evolution in the Neotropics. The “origin of Neotropical biodiversity” encapsulates at least two contrasting subjects: the timing of origin of hyperdiversity (i.e., “when did the Neotropics reach globally outstanding levels of species richness?”), and the actual age of extant species (i.e., “when did the species that we see today split from their most recent common ancestors, as defined by their stem age?”; Hoorn et al., 2011). It is clear that there have been unusual periods of time throughout geological history, both in terms of biotic and abiotic events (Jaramillo, 2006; Hoorn et al., 2010b; Jaramillo et al., 2010). However, all periods of time have contributed to the current biodiversity, if seen in the perspective of the “evolutionary continuum” that bridges the core biodiversity disciplines (Fig. 4).
Examples of studies that have sought for “special” periods of time often come from time-calibrated molecular phylogenies. For instance, butterfly species-pairs seem to be relatively young in origin (i.e., <2 Ma), suggesting that the Pleistocene and Holocene may have represented “extraordinary times” for Neotropical butterfly speciation (Garzón-Orduña, Benetti Longhini & Brower, 2014) and following the refugium theory (Table 4). However, time-calibrated phylogenies may not fully address the potential impact of extinction and species duration (Hoorn et al., 2011). In other words, if we were able to travel back in time to any period and sequence species around us, the odds are that most species alive might also be around two Ma old or less. In addition, the definition of “species” may vary considerably depending on data source (e.g., based on the fossil record and extant populations) and across taxa. Highly structured populations with considerable genetic divergences may be seen as “incipient species” that have not yet completed the speciation process (Craig et al., 2018). Variable species concepts and adequate sampling of extant and extinct taxa represent a serious barrier for our understanding of Neotropical diversification (Tables 1–3 and 5).
Advancing Neotropical research
Comparative biology has experienced major advancements in the theory and practice of biogeography and molecular phylogenetics during the past decades. However, we still need to increase sampling of organisms drastically in order to advance our knowledge on the patterns and processes underlying Neotropical biodiversity (Feeley, 2015). However, fieldwork in the Neotropics, especially in pristine areas, is time consuming and logistically demanding. Research funding for exploratory inventory projects is also becoming increasingly harder to obtain, despite the fact that highly successful projects (i.e., sequencing the first human genome and creating the Amazon Tree Diversity Network) were initially discovery-driven, rather than focused on testing specific hypotheses. Furthermore, obtaining permits to collect and export biological samples is also challenging, involving many differences across national legislations. Finally, some authors have seen the need of fieldwork as less relevant in this era of museomics (Buerki & Baker, 2016; Zedane et al., 2016).
Despite all these obstacles, fieldwork remains absolutely essential for biodiversity data generation and monitoring (Albert, 2002). Fieldwork also provides students and researchers with a deeper understanding of their study systems, often providing new ideas and questions, while facilitating the establishment of new collaborations, enabling the exchange of knowledge, fueling the development of new methods, and increasing the possibilities of major discoveries (Fleischner et al., 2017). We should seriously consider new strategies for the generation of new biodiversity data, as well as for the syntheses of the already available data.
Multi-taxon field campaigns could provide unique opportunities for intensive sampling, while optimizing resources, bureaucratic, and logistic efforts. However, this vision requires a radical re-thinking and re-organization. We need to provide young generations with the training, tools and resources needed to carry out research on all aspects of biodiversity. We also need to support taxonomic specialists and institutions in order to adequately study, archive and facilitate free access to biological collections and associated data. In addition, we also need to join forces across nations and disciplines for mutual benefit and joint scientific growth. Clearly, these investments would be worthwhile from a global perspective. The future of Neotropical biodiversity research depends on extensive collaborations and coordinated efforts.
Take-Home Messages
Five main take home messages can be taken from this review, namely:
Neotropical biodiversity is exceedingly high, regardless of the axis explored (e.g., taxonomic, phylogenetic, functional, ecosystems);
Understanding the origins, evolution, maintenance, and distribution patterns of Neotropical biodiversity is a grand scientific challenge with many remaining unknowns;
“Trans-disciplinary biogeography” aims to better integrate the seemingly disparate disciplines required to explore the biotic and abiotic evolution of the Neotropics;
Many methodological advances will be required to deal with the increasing wealth of biodiversity data and associated environmental and geological variables;
There is an urgent need to fill the many gaps in biodiversity knowledge, including extant and extinct taxa and their interactions. This calls for a “renaissance” for fieldwork.
Acknowledgments
This paper is a product of the “Origin of Biodiversity Workshop” organized by Chalmers University of Technology and the University of Gothenburg, under the auspices of the Gothenburg Centre for Advanced Studies (GoCAS). We are particularly grateful to GoCAS organizers Karin Hårding, Gunnar Nyman, and Mattias Marklund for their continuous support and assistance. We thank Juan E. Guevara, Thomas J. Givnish, Luis F. Aguirre, Tomas Hrbek, Julissa Roncal, and the editor Stephen Johnson for helpful suggestions on the pre-print of this paper. We also thank Tomas Hrbek for handling and editing the peer-reviewed manuscript, and two anonymous reviewers for constructive comments.
Funding Statement
The workshop “Origins of Biodiversity” was funded by Chalmers University of Technology and the University of Gothenburg. The following researchers are supported by scholarship or research grants from the following agencies: Alexandre Antonelli by the Swedish Research Council (B0569601), the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013, ERC Grant Agreement 331024), the Swedish Foundation for Strategic Research, a Wallenberg Academy Fellowship, the Faculty of Sciences at the University of Gothenburg, the Wenner-Gren Foundations, and the David Rockefeller Center for Latin American Studies at Harvard University; Camila D. Ritter by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 249064/2013-8); Daniele Silvestro by the Swedish Research Council (2015-04748); Fernanda P. Werneck by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, the Fundação de Amparo à Pesquisa do Amazonas, the Partnerships for Enhanced Engagement in Research from the U.S. National Academy of Sciences (PEER NAS/USAID, USA), and the L’Oréal-UNESCO for Women in Science Awards (Brazil and France); Isabel Sanmartín by MINECO/FEDER (CGL2015-67849-P); James S. Albert by the National Science Foundation (NSF 0614334, 0741450, and 1354511); Josué A. R. Azevedo by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (99999.001292/2015-03); Lúcia G. Lohmann by a collaborative Dimensions of Biodiversity-NSF/Biota-FAPESP grant (FAPESP 2012/50260-6) and by the Fundação de Amparo à Pesquisa do Estado de São Paulo (2017/12797-1); Pável Matos-Maraví by a Marie Skłodowska-Curie fellowship (704035); Thaís Guedes by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2013/04170-8 and 2014/18837-7). No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Scott V. Edwards is an Academic Editor for PeerJ.
Author Contributions
Alexandre Antonelli conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
María Ariza prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, formatted references and merged the manuscript revisions.
James Albert prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Tobias Andermann prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Josué Azevedo prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Christine Bacon prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Søren Faurby analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Thais Guedes prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Carina Hoorn prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Lúcia G. Lohmann prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Pável Matos-Maraví prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Camila D. Ritter prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Isabel Sanmartín prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Daniele Silvestro prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Marcelo Tejedor prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hans ter Steege prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hanna Tuomisto prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Fernanda P. Werneck prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Alexander Zizka analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Scott V. Edwards conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The research in this literature review did not generate any data or code.
References
- Aguilée, Claessen & Lambert (2013).Aguilée R, Claessen D, Lambert A. Adaptive radiation driven by the interplay of eco-evolutionary and landscape dynamics. Evolution. 2013;67(5):1291–1306. doi: 10.1111/evo.12008. [DOI] [PubMed] [Google Scholar]
- Albert (2002).Albert JS. Eternal vigilance on an Amazon floodplain. Current Biology. 2002;12(13):R442–R443. doi: 10.1016/S0960-9822(02)00937-5. [DOI] [PubMed] [Google Scholar]
- Albert et al. (2011).Albert JS, Carvalho TP, Petry P, Holder MA, Maxime EL, Espino J, Corahua I, Quispe R, Rengifo B, Ortega H, Reis RE. Aquatic biodiversity in the Amazon: habitat specialization and geographic isolation promote species richness. Animals. 2011;1(2):205–241. doi: 10.3390/ani1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert et al. (2018).Albert JS, Craig JM, Tagliacollo VA, Petry P. Upland and lowland fishes: a test of the river capture hypothesis. In: Hoorn C, Antonelli A, Antonelli A, editors. Mountains, Climate and Biodiversity. New York: Wiley-Blackwell; 2018. pp. 273–294. [Google Scholar]
- Albert, Petry & Reis (2011).Albert JS, Petry P, Reis RE. Major biogeographic and phylogenetic patterns. In: Albert JS, Reis RE, editors. Historical Biogeography of Neotropical Freshwater Fishes. Berkeley: University of California Press; 2011. pp. 21–57. [Google Scholar]
- Albert et al. (2017).Albert JS, Schoolmaster DR, Tagliacollo V, Duke-Sylvester SM. Barrier displacement on a neutral landscape: toward a theory of continental biogeography. Systematic Biology. 2017;66(2):167–182. doi: 10.1093/sysbio/syw080. [DOI] [PubMed] [Google Scholar]
- Albert, Val & Hoorn (in press).Albert JS, Val P, Hoorn C. The changing course of the Amazon in the Neogene: Center stage for Neotropical diversification. Neotropical Ichthyology. in press [Google Scholar]
- Alonso, Metz & Fine (2013).Alonso JÁ, Metz MR, Fine PVA. Habitat specialization by birds in Western Amazonian white-sand forests. Biotropica. 2013;45(3):365–372. [Google Scholar]
- Arrington & Winemiller (2009).Arrington DA, Winemiller KO. Habitat affinity, the seasonal flood pulse, and community assembly in the littoral zone of a Neotropical floodplain river. Journal of the North American Benthological Society. 2009;25(1):126–141. doi: 10.1899/0887-3593(2006)25[126:HATSFP]2.0.CO;2. [DOI] [Google Scholar]
- Andújar et al. (2015).Andújar C, Arribas P, Ruzicka F, Crampton-Platt A, Timmermans MJTN, Vogler AP. Phylogenetic community ecology of soil biodiversity using mitochondrial metagenomics. Molecular Ecology. 2015;24(14):3603–3617. doi: 10.1111/mec.13195. [DOI] [PubMed] [Google Scholar]
- Ané et al. (2007).Ané C, Larget B, Baum DA, Smith SD, Rokas A. Bayesian estimation of concordance among gene trees. Molecular Biology and Evolution. 2007;24(7):1575. doi: 10.1093/molbev/msm107. [DOI] [PubMed] [Google Scholar]
- Antonelli (2017a).Antonelli A. Biogeography: drivers of bioregionalization. Nature Ecology & Evolution. 2017a;1(4):0114. doi: 10.1038/s41559-017-0114. [DOI] [PubMed] [Google Scholar]
- Antonelli (2017b).Antonelli A. Comparative biogeography, big data, and common myths. In: Friis I, Balslev H, editors. Tropical plant collections: legacies from the past? Essential tools for the future? Vol. 6. Copenhagen: Royal Danish Academy of Sciences and Letters; 2017b. Scientia Danica B (Biological) [Google Scholar]
- Antonelli et al. (2009).Antonelli A, Nylander JAA, Persson C, Sanmartín I. Tracing the impact of the Andean uplift on Neotropical plant evolution. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(24):9749–9754. doi: 10.1073/pnas.0811421106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli & Sanmartín (2011).Antonelli A, Sanmartín I. Why are there so many plant species in the Neotropics? Taxon. 2011;60(2):403–414. [Google Scholar]
- Antonelli et al. (2018).Antonelli A, Zizka A, Antunes Carvalho F, Scharn R, Bacon CD, Silvestro D, Condamine FL. Amazonia is the primary source of Neotropical biodiversity. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(23):6034–6039. doi: 10.1073/pnas.1713819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour & López-Fernández (2014).Arbour JH, López-Fernández HS. Adaptive landscape and functional diversity of Neotropical cichlids: implications for the ecology and evolution of Cichlinae (Cichlidae; Cichliformes) Journal of Evolutionary Biology. 2014;27(11):2431–2442. doi: 10.1111/jeb.12486. [DOI] [PubMed] [Google Scholar]
- Arellano et al. (2016).Arellano G, Tello JS, Jørgensen PM, Fuentes AF, Loza MI, Torrez V, Macía MJ. Disentangling environmental and spatial processes of community assembly in tropical forests from local to regional scales. Oikos. 2016;125(3):326–335. doi: 10.1111/oik.02426. [DOI] [Google Scholar]
- Arias (2017).Arias JS. An event model for phylogenetic biogeography using explicitly geographical ranges. Journal of Biogeography. 2017;44(10):2225–2235. doi: 10.1111/jbi.13024. [DOI] [Google Scholar]
- Arias, Szumik & Goloboff (2011).Arias JS, Szumik CA, Goloboff PA. Spatial analysis of vicariance: a method for using direct geographical information in historical biogeography. Cladistics. 2011;27(6):617–628. doi: 10.1111/j.1096-0031.2011.00353.x. [DOI] [PubMed] [Google Scholar]
- Armbruster, Van Der Sleen & Lujan (2018).Armbruster J, Van Der Sleen P, Lujan N. Family Loricariidae—Suckermouth armoured catfishes. In: Van Der Sleen P, Albert JS, editors. Field Guide to the Fishes of the Amazon, Orinoco and Guianas. New Jersey: Princeton University Press; 2018. pp. 253–258. [Google Scholar]
- Azevedo, Valdujo & Nogueira (2016).Azevedo JAR, Valdujo PH, Nogueira C De C. Biogeography of anurans and squamates in the Cerrado hotspot: coincident endemism patterns in the richest and most impacted savanna on the globe. Journal of Biogeography. 2016;43(12):2454–2464. doi: 10.1111/jbi.12803. [DOI] [Google Scholar]
- Bacon (2013).Bacon CD. Biome evolution and biogeographical change through time. Frontiers of Biogeography. 2013;5(4):227–231. [Google Scholar]
- Bacon, Baker & Simmons (2012).Bacon CD, Baker WJ, Simmons MP. Miocene dispersal drives island radiations in the palm tribe Trachycarpeae (Arecaceae) Systematic Biology. 2012;61(3):426–442. doi: 10.1093/sysbio/syr123. [DOI] [PubMed] [Google Scholar]
- Bacon et al. (2015).Bacon CD, Silvestro D, Jaramillo CA, Smith BT, Chakrabarty P, Antonelli A. Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(19):6110–6115. doi: 10.1073/pnas.1423853112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon et al. (2018).Bacon CD, Velásquez-Puentes FJ, Hoorn C, Antonelli A. Iriarteeae palms tracked the uplift of Andean Cordilleras. Journal of Biogeography. 2018;45(7):1653–1663. doi: 10.1111/jbi.13350. [DOI] [Google Scholar]
- Badgley et al. (2017).Badgley C, Smiley TM, Terry R, Davis EB, DeSantis LRG, Fox DL, Hopkins SSB, Jezkova T, Matocq MD, Matzke N, McGuire JL, Mulch A, Riddle BR, Louise Roth V, Samuels JX, Strömberg CAE, Yanites BJ. Biodiversity and topographic complexity: modern and geohistorical perspectives. Trends in Ecology & Evolution. 2017;32(3):211–226. doi: 10.1016/j.tree.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker & Couvreur (2012).Baker WJ, Couvreur TLP. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. Journal of Biogeography. 2012;40(2):274–285. doi: 10.1111/j.1365-2699.2012.02795.x. [DOI] [Google Scholar]
- Baker et al. (2014).Baker PA, Fritz SC, Dick CW, Eckert AJ, Horton BK, Manzoni S, Ribas CC, Garzione CN, Battisti DS. The emerging field of geogenomics: constraining geological problems with genetic data. Earth-Science Reviews. 2014;135:38–47. doi: 10.1016/j.earscirev.2014.04.001. [DOI] [Google Scholar]
- Basset et al. (2012).Basset Y, Cizek L, Cuénoud P, Didham RK, Guilhaumon F, Missa O, Novotny V, Ødegaard F, Roslin T, Schmidl J, Tishechkin AK, Winchester NN, Roubik DW, Aberlenc H-P, Bail J, Barrios H, Bridle JR, Castaño-Meneses G, Corbara B, Curletti G, Da Rocha WD, De Bakker D, Delabie JHC, Dejean A, Fagan LL, Floren A, Kitching RL, Medianero E, Miller SE, De Oliveira EG, Orivel J, Pollet M, Rapp M, Ribeiro SP, Roisin Y, Schmidt JB, Sørensen LL, Leponce M. Arthropod diversity in a tropical forest. Science. 2012;338(6113):1481–1484. doi: 10.1126/science.1226727. [DOI] [PubMed] [Google Scholar]
- Bastazini (2017).Bastazini VAG. Untangling the tangled bank: a novel method for partitioning the effects of phylogenies and traits on ecological networks. Evolutionary Biology. 2017;44(3):312–324. doi: 10.1007/s11692-017-9409-8. [DOI] [Google Scholar]
- Beck (2006).Beck H. A review of peccary–palm interactions and their ecological ramifications across the Neotropics. Journal of Mammalogy. 2006;87(3):519–530. doi: 10.1644/05-MAMM-A-174R1.1. [DOI] [Google Scholar]
- Bello et al. (2015).Bello C, Galetti M, Pizo MA, Magnago LFS, Rocha MF, Lima RAF, Peres CA, Ovaskainen O, Jordano P. Defaunation affects carbon storage in tropical forests. Science Advances. 2015;1(11):e1501105. doi: 10.1126/sciadv.1501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemmels et al. (2018).Bemmels JB, Wright SJ, Garwood NC, Queenborough SA, Valencia R, Dick CW. Filter-dispersal assembly of lowland Neotropical rainforests across the Andes. Ecography. 2018;6:1. doi: 10.1111/ecog.03473. [DOI] [Google Scholar]
- Benavides et al. (2005).Benavides AM, Duque A, Duivenvoorden JF, Vasco A, Callejas R. A first quantitative census of vascular epiphytes in rain forests of Colombian Amazonia. Biodiversity and Conservation. 2005;14(3):739–758. doi: 10.1007/s10531-004-3920-9. [DOI] [Google Scholar]
- Bertrand, Pleijel & Rouse (2006).Bertrand Y, Pleijel F, Rouse GW. Taxonomic surrogacy in biodiversity assessments, and the meaning of Linnaean ranks. Systematics and Biodiversity. 2006;4(2):149–159. doi: 10.1017/S1477200005001908. [DOI] [Google Scholar]
- Bickford et al. (2007).Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution. 2007;22(3):148–155. doi: 10.1016/j.tree.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Blisniuk et al. (2005).Blisniuk PM, Stern LA, Page Chamberlain C, Idleman B, Zeitler PK. Climatic and ecologic changes during Miocene surface uplift in the Southern Patagonian Andes. Earth and Planetary Science Letters. 2005;230(1–2):125–142. doi: 10.1016/j.epsl.2004.11.015. [DOI] [Google Scholar]
- Bond (2005).Bond WJ. Large parts of the world are brown or black: a different view on the “Green World” hypothesis. Journal of Vegetation Science. 2005;16(3):261. doi: 10.1658/1100-9233(2005)016[0261:lpotwa]2.0.co;2. [DOI] [Google Scholar]
- Boucher (1988).Boucher DH. The Biology of Mutualism: Ecology and Evolution. Oxford: Oxford University Press; 1988. The idea of mutualism, past and future; p. 388. [Google Scholar]
- Bozdogan (1987).Bozdogan H. Model selection and Akaike’s Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika. 1987;52(3):345–370. doi: 10.1007/bf02294361. [DOI] [Google Scholar]
- Bravo et al. (2018).Bravo GA, Antonelli A, Bacon CD, Bartoszek K, Blom M, Huynh S, Jones G, Knowles LL, Lamichhaney S, Marcussen T, Morlon H, Nakhleh L, Oxelman B, Pfeil B, Schliep A, Wahlberg N, Werneck F, Wiedenhoeft J, Willows-Munro S, Edwards SV. Embracing heterogeneity: building the tree of life and the future of phylogenomics. PeerJ. 2018;6:e26449v3. doi: 10.7717/peerj.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm, Colwell & Kluge (2007).Brehm G, Colwell RK, Kluge J. The role of environment and mid-domain effect on moth species richness along a tropical elevational gradient. Global Ecology and Biogeography. 2007;16(2):205–219. doi: 10.1111/j.1466-8238.2006.00281.x. [DOI] [Google Scholar]
- Brown & Svenning (2013).Brown JH, Svenning J-C. Why are there so many species in the tropics? Journal of Biogeography. 2013;41(1):8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, Stachowicz & Bertness (2003).Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution. 2003;18(3):119–125. doi: 10.1016/S0169-5347(02)00045-9. [DOI] [Google Scholar]
- Buerki & Baker (2016).Buerki S, Baker WJ. Collections-based research in the genomic era. Biological Journal of the Linnean Society. 2016;117(1):5–10. doi: 10.1111/bij.12721. [DOI] [Google Scholar]
- Burns, Unitt & Mason (2016).Burns KJ, Unitt P, Mason NA. A genus-level classification of the family Thraupidae (Class Aves: Order Passeriformes) Zootaxa. 2016;4088(3):329. doi: 10.11646/zootaxa.4088.3.2. [DOI] [PubMed] [Google Scholar]
- Byrne & Hopper (2008).Byrne M, Hopper SD. Granite outcrops as ancient islands in old landscapes: evidence from the phylogeography and population genetics of Eucalyptus caesia (Myrtaceae) in Western Australia. Biological Journal of the Linnean Society. 2008;93(1):177–188. doi: 10.1111/j.1095-8312.2007.00946.x. [DOI] [Google Scholar]
- Cabral, Valente & Hartig (2017).Cabral JS, Valente L, Hartig F. Mechanistic simulation models in macroecology and biogeography: state-of-art and prospects. Ecography. 2017;40(2):267–280. doi: 10.1111/ecog.02480. [DOI] [Google Scholar]
- Cámara-Leret et al. (2017).Cámara-Leret R, Tuomisto H, Ruokolainen K, Balslev H, Munch Kristiansen S. Modelling responses of western Amazonian palms to soil nutrients. Journal of Ecology. 2017;105(2):367–381. doi: 10.1111/1365-2745.12708. [DOI] [Google Scholar]
- Camargo et al. (2013).Camargo A, Werneck FP, Morando M, Sites JW, Avila LJ. Quaternary range and demographic expansion of Liolaemus darwinii (Squamata: Liolaemidae) in the Monte Desert of Central Argentina using Bayesian phylogeography and ecological niche modelling. Molecular Ecology. 2013;22(15):4038–4054. doi: 10.1111/mec.12369. [DOI] [PubMed] [Google Scholar]
- Cardoso et al. (2017).Cardoso D, Särkinen T, Alexander S, Amorim AM, Bittrich V, Celis M, Daly DC, Fiaschi P, Funk VA, Giacomin LL, Goldenberg R, Heiden G, Iganci J, Kelloff CL, Knapp S, Cavalcante De Lima H, Machado AFP, Dos Santos RM, Mello-Silva R, Michelangeli FA, Mitchell J, Moonlight P, De Moraes PLR, Mori SA, Nunes TS, Pennington TD, Pirani JR, Prance GT, De Queiroz LP, Rapini A, Riina R, Rincon CAV, Roque N, Shimizu G, Sobral M, Stehmann JR, Stevens WD, Taylor CM, Trovó M, Van Den Berg C, Van Der Werff H, Viana PL, Zartman CE, Forzza RC. Amazon plant diversity revealed by a taxonomically verified species list. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(40):10695–10700. doi: 10.1073/pnas.1706756114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaval et al. (2009).Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C. Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science. 2009;323(5915):785–789. doi: 10.1126/science.1166955. [DOI] [PubMed] [Google Scholar]
- Carnaval & Moritz (2008).Carnaval AC, Moritz C. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. Journal of Biogeography. 2008;35(7):1187–1201. doi: 10.1111/j.1365-2699.2007.01870.x. [DOI] [Google Scholar]
- Carnaval et al. (2014).Carnaval AC, Waltari E, Rodrigues MT, Rosauer D, VanDerWal J, Damasceno R, Prates I, Strangas M, Spanos Z, Rivera D, Pie MR, Firkowski CR, Bornschein MR, Ribeiro LF, Moritz C. Prediction of phylogeographic endemism in an environmentally complex biome. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1792):20141461. doi: 10.1098/rspb.2014.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagranda, Roig-Juñet & Szumik (2009).Casagranda MD, Roig-Juñet S, Szumik C. Endemismo a diferentes escalas espaciales: un ejemplo con Carabidae (Coleóptera: Insecta) de América del Sur austral. Revista chilena de historia natural. 2009;82:17–42. doi: 10.4067/S0716-078X2009000100002. [DOI] [Google Scholar]
- Chazot et al. (2016).Chazot N, Willmott KR, Condamine FL, De-Silva DL, Freitas AVL, Lamas G, Morlon H, Giraldo CE, Jiggins CD, Joron M, Mallet J, Uribe S, Elias M. Into the Andes: multiple independent colonizations drive montane diversity in the Neotropical clearwing butterflies Godyridina. Molecular Ecology. 2016;25(22):5765–5784. doi: 10.1111/mec.13773. [DOI] [PubMed] [Google Scholar]
- Chao & Jost (2012).Chao A, Jost L. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology. 2012;93(12):2533–2547. doi: 10.1890/11-1952.1. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2013).Cheng H, Sinha A, Cruz FW, Wang X, Edwards RL, d’Horta FM, Ribas CC, Vuille M, Stott LD, Auler AS. Climate change patterns in Amazonia and biodiversity. Nature Communications. 2013;4(1):1411–1416. doi: 10.1038/ncomms2415. [DOI] [PubMed] [Google Scholar]
- Chomicki & Renner (2015).Chomicki G, Renner SS. Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytologist. 2015;207(2):411–424. doi: 10.1111/nph.13271. [DOI] [PubMed] [Google Scholar]
- Chomicki, Ward & Renner (2015).Chomicki G, Ward PS, Renner SS. Macroevolutionary assembly of ant/plant symbioses: Pseudomyrmex ants and their ant-housing plants in the Neotropics. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1819):20152200–20152209. doi: 10.1098/rspb.2015.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cione et al. (2005).Cione AL, De Las Mercedes Azpelicueta M, Casciotta JR, Dozo MT. Tropical freshwater teleosts from Miocene beds of eastern Patagonia, southern Argentina. Geobios. 2005;38(1):29–42. doi: 10.1016/j.geobios.2003.08.005. [DOI] [Google Scholar]
- Clarke, Thomas & Freckleton (2016).Clarke M, Thomas GH, Freckleton RP. Trait evolution in adaptive radiations: modeling and measuring interspecific competition on phylogenies. The American Naturalist. 2016;189(2):121–137. doi: 10.1086/689819. [DOI] [PubMed] [Google Scholar]
- Clinebell et al. (1995).Clinebell RR, Phillips OL, Gentry AH, Stark N, Zuuring H. Prediction of neotropical tree and liana species richness from soil and climatic data. Biodiversity and Conservation. 1995;4(1):56–90. doi: 10.1007/BF00115314. [DOI] [Google Scholar]
- Coddington et al. (2009).Coddington JA, Agnarsson I, Miller JA, Kuntner M, Hormiga G. Undersampling bias: the null hypothesis for singleton species in tropical arthropod surveys. Journal of Animal Ecology. 2009;78(3):573–584. doi: 10.1111/j.1365-2656.2009.01525.x. [DOI] [PubMed] [Google Scholar]
- Colli et al. (2016).Colli GR, Fenker J, Tedeschi LG, Barreto-Lima AF, Mott T, Ribeiro SLB. In the depths of obscurity: knowledge gaps and extinction risk of Brazilian worm lizards (Squamata, Amphisbaenidae) Biological Conservation. 2016;204:51–62. doi: 10.1016/j.biocon.2016.07.033. [DOI] [Google Scholar]
- Condamine et al. (2018).Condamine FL, Antonelli A, Lagomarsino LP, Hoorn C, Liow LH. Teasing apart mountain uplift, climate change and biotic drivers of species diversification. In: Hoorn C, Antonelli A, Perrigo A, editors. Mountains, Climate and Biodiversity. New York: Wiley-Blackwell; 2018. pp. 257–272. [Google Scholar]
- Connolly et al. (2017).Connolly SR, Keith SA, Colwell RK, Rahbek C. Process, Mechanism, and Modeling in Macroecology. Trends in Ecology & Evolution. 2017;32(11):835–844. doi: 10.1016/j.tree.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Correa Restrepo et al. (2016).Correa Restrepo Z, Núñez Avellaneda LA, González-Caro S, Velásquez-Puentes FJ, Bacon CD. Exploring palm-insect interactions across geographical and environmental gradients. Botanical Journal of the Linnean Society. 2016;182(2):389–397. doi: 10.1111/boj.12443. [DOI] [Google Scholar]
- Costa et al. (2009a).Costa FRC, Guillaumet J-L, Lima AP, Pereira OS. Gradients within gradients: The mesoscale distribution patterns of palms in a central Amazonian forest. Journal of Vegetation Science. 2009a;20(1):69–78. doi: 10.1111/j.1654-1103.2009.05314.x. [DOI] [Google Scholar]
- Costa et al. (2017).Costa GC, Hampe A, Ledru M-P, Martinez PA, Mazzochini GG, Shepard DB, Werneck FP, Moritz C, Carnaval AC. Biome stability in South America over the last 30 kyr: Inferences from long-term vegetation dynamics and habitat modelling. Global Ecology and Biogeography. 2017;27(3):285–297. doi: 10.1111/geb.12694. [DOI] [Google Scholar]
- Costa, Magnusson & Luizao (2005).Costa FRC, Magnusson WE, Luizao RC. Mesoscale distribution patterns of Amazonian understorey herbs in relation to topography, soil and watersheds. Journal of Ecology. 2005;93(5):863–878. doi: 10.1111/j.1365-2745.2005.01020.x. [DOI] [Google Scholar]
- Costa et al. (2007).Costa GC, Nogueira C, Machado RB, Colli GR. Squamate richness in the Brazilian Cerrado and its environmental-climatic associations. Diversity and Distributions. 2007;13(6):714–724. doi: 10.1111/j.1472-4642.2007.00369.x. [DOI] [Google Scholar]
- Costa et al. (2009b).Costa GC, Nogueira C, Machado RB, Colli GR. Sampling bias and the use of ecological niche modeling in conservation planning: a field evaluation in a biodiversity hotspot. Biodiversity and Conservation. 2009b;19(3):883–899. doi: 10.1007/s10531-009-9746-8. [DOI] [Google Scholar]
- Cowie & Holland (2006).Cowie RH, Holland BS. Dispersal is fundamental to biogeography and the evolution of biodiversity on oceanic islands. Journal of Biogeography. 2006;33(2):193–198. doi: 10.1111/j.1365-2699.2005.01383.x. [DOI] [Google Scholar]
- Craig et al. (2018).Craig JM, Malabarba LR, Crampton WGR, Albert JS. Revision of banded knifefishes of the Gymnotus carapo and G. tigre clades (Gymnotidae Gymnotiformes) from the Southern Neotropics. Zootaxa. 2018;4379(1):47–73. doi: 10.11646/zootaxa.4379.1.3. [DOI] [PubMed] [Google Scholar]
- Craw et al. (2016).Craw D, Upton P, Burridge CP, Wallis GP, Waters JM. Rapid biological speciation driven by tectonic evolution in New Zealand. Nature Geoscience. 2016;9(2):140–144. doi: 10.1038/ngeo2618. [DOI] [Google Scholar]
- Crisci et al. (1991).Crisci JV, Cigliano MM, Morrone JJ, Roig-Juñet S. Historical biogeography of southern South America. Systematic Biology. 1991;40(2):152–171. doi: 10.1093/sysbio/40.2.152. [DOI] [Google Scholar]
- Cruz-Neto, Garland & Abe (2001).Cruz-Neto AP, Garland T, Abe AS. Diet, phylogeny, and basal metabolic rate in phyllostomid bats. Zoology. 2001;104(1):49–58. doi: 10.1078/0944-2006-00006. [DOI] [PubMed] [Google Scholar]
- Ribeiro (1999).Ribeiro JEL Da S. Flora da Reserva Ducke: guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazônia Central. Manaus: INPA; 1999. [Google Scholar]
- Dagosta & De Pinna (2017).Dagosta FCP, De Pinna M. Biogeography of Amazonian fishes: deconstructing river basins as biogeographic units. Neotropical Ichthyology. 2017;15(3):1–24. doi: 10.1590/1982-0224-20170034. [DOI] [Google Scholar]
- De Baets, Antonelli & Donoghue (2016).De Baets K, Antonelli A, Donoghue PCJ. Tectonic blocks and molecular clocks. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2016;371(1699):20160098. doi: 10.1098/rstb.2016.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Queiroz (2005).De Queiroz K. A unified concept of species and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences. 2005;56(18):196–215. [Google Scholar]
- DeAngelis & Grimm (2017).DeAngelis DL, Grimm V. Individual-based models in ecology after four decades. F1000Prime Reports. 2017;6:39. doi: 10.12703/P6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter et al. (2017).Dexter KG, Lavin M, Torke BM, Twyford AD, Kursar TA, Coley PD, Drake C, Hollands R, Pennington RT. Dispersal assembly of rain forest tree communities across the Amazon basin. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(10):2645–2650. doi: 10.1073/pnas.1613655114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados & Barber (2017).Diazgranados M, Barber JC. Geography shapes the phylogeny of frailejones (Espeletiinae Cuatrec., Asteraceae): a remarkable example of recent rapid radiation in sky islands. PeerJ. 2017;5:e2968. doi: 10.7717/peerj.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz-Filho et al. (2011).Diniz-Filho JAF, Siqueira T, Padial AA, Rangel TF, Landeiro VL, Bini LM. Spatial autocorrelation analysis allows disentangling the balance between neutral and niche processes in metacommunities. Oikos. 2011;121(2):201–210. doi: 10.1111/j.1600-0706.2011.19563.x. [DOI] [Google Scholar]
- Doughty, Faurby & Svenning (2016).Doughty CE, Faurby S, Svenning J-C. The impact of the megafauna extinctions on savanna woody cover in South America. Ecography. 2016;39(2):213–222. doi: 10.1111/ecog.01593. [DOI] [Google Scholar]
- Dransfield et al. (2008).Dransfield J, Uhl NW, Connie AB, Baker WJ, Harley MM, Lewis CE. Genera Palmarum: The Evolution and Classification of Palms. Kew: Royal Botanic Gardens; 2008. [Google Scholar]
- Duellman (1989).Duellman WE. Tropical herpetofaunal communities: patterns of community structure in neotropical rainforests. In: Harmelin-Vivien ML, Bourliére F, editors. Vertebrates in Complex Tropical Systems. Ecological Studies. New York: Springer; 1989. pp. 61–88. [Google Scholar]
- Edler et al. (2016).Edler D, Guedes T, Zizka A, Rosvall M, Antonelli A. Infomap bioregions: interactive mapping of biogeographical regions from species distributions. Systematic Biology. 2016;66(2):197–204. doi: 10.1093/sysbio/syw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldredge (1989).Eldredge N. Time frames: the evolution of punctuated equilibria. New Jersey: Princeton University Press; 1989. [Google Scholar]
- Emerson & Kolm (2005).Emerson BC, Kolm N. Species diversity can drive speciation. Nature. 2005;434(7036):1015–1017. doi: 10.1038/nature03450. [DOI] [PubMed] [Google Scholar]
- Espeland et al. (2015).Espeland M, Hall JPW, DeVries PJ, Lees DC, Cornwall M, Hsu Y-F, Wu L-W, Campbell DL, Talavera G, Vila R, Salzman S, Ruehr S, Lohman DJ, Pierce NE. Ancient Neotropical origin and recent recolonisation: phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea) Molecular Phylogenetics and Evolution. 2015;93:296–306. doi: 10.1016/j.ympev.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Esquivel-Muelbert et al. (2017).Esquivel-Muelbert A, Baker TR, Dexter KG, Lewis SL, Ter Steege H, Lopez-Gonzalez G, Monteagudo Mendoza A, Brienen R, Feldpausch TR, Pitman N, Alonso A, Van Der Heijden G, Peña-Claros M, Ahuite M, Alexiaides M, Álvarez Dávila E, Murakami AA, Arroyo L, Aulestia M, Balslev H, Barroso J, Boot R, Cano A, Chama Moscoso V, Comiskey JA, Cornejo F, Dallmeier F, Daly DC, Dávila N, Duivenvoorden JF, Duque Montoya AJ, Erwin T, Di Fiore A, Fredericksen T, Fuentes A, García-Villacorta R, Gonzales T, Guevara Andino JE, Honorio Coronado EN, Huamantupa-Chuquimaco I, Maria Jiménez RE, Killeen TJ, Malhi Y, Mendoza C, Mogollón H, Jørgensen PM, Montero JC, Mostacedo B, Nauray W, Neill D, Vargas PN, Palacios S, Palacios Cuenca W, Pallqui Camacho NC, Peacock J, Phillips JF, Pickavance G, Quesada CA, Ramirez-Angulo H, Restrepo Z, Reynel Rodriguez C, Paredes MR, Peñuela-Mora MC, Sierra R, Silveira M, Stevenson P, Stropp J, Terborgh J, Tirado M, Toledo M, Torres-Lezama A, Umaña MN, Urrego LE, Vasquez Martinez R, Gamarra LV, Vela CIA, Vilanova Torre E, Vos V, Von Hildebrand P, Vriesendorp C, Wang O, Young KR, Zartman CE, Phillips OL. Seasonal drought limits tree species across the Neotropics. Ecography. 2017;40(5):618–629. doi: 10.1111/ecog.01904. [DOI] [Google Scholar]
- Faith (1992).Faith DP. Conservation evaluation and phylogenetic diversity. Biological Conservation. 1992;61(1):1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- Faurby, Eiserhardt & Svenning (2016).Faurby S, Eiserhardt WL, Svenning J-C. Strong effects of variation in taxonomic opinion on diversification analyses. Methods in Ecology and Evolution. 2016;7(1):4–13. doi: 10.1111/2041-210X.12449. [DOI] [Google Scholar]
- Faurby & Svenning (2015).Faurby S, Svenning J-C. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Diversity and Distributions. 2015;21(10):1155–1166. doi: 10.1111/ddi.12369. [DOI] [Google Scholar]
- Feeley (2015).Feeley KJ. Are we filling the data void? An assessment of the amount and extent of plant collection records and census data available for tropical South America. PLOS ONE. 2015;10(4):e0125629. doi: 10.1371/journal.pone.0125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenker et al. (2014).Fenker J, Tedeschi LG, Pyron RA, Nogueira C de C. Phylogenetic diversity, habitat loss and conservation in South American pitvipers (Crotalinae: Bothrops and Bothrocophias) Diversity and Distributions. 2014;20(10):1108–1119. doi: 10.1111/ddi.12217. [DOI] [Google Scholar]
- Field et al. (2009).Field R, Hawkins BA, Cornell HV, Currie DJ, Filho JAFD, Guégan JF, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O’Brien EM, Turner JRG. Spatial species-richness gradients across scales: a meta-analysis. Journal of Biogeography. 2009;36(1):132–147. doi: 10.1111/j.1365-2699.2008.01963.x. [DOI] [Google Scholar]
- Figueiredo et al. (2009).Figueiredo J, Hoorn C, Van Der Ven P, Soares E. Late Miocene onset of the Amazon river and the Amazon deep-sea fan: evidence from the Foz do Amazonas basin. Geology. 2009;37(7):619–622. doi: 10.1130/G25567A.1. [DOI] [Google Scholar]
- Figueiredo et al. (2017).Figueiredo FOG, Zuquim G, Tuomisto H, Moulatlet GM, Balslev H, Costa FRC. Beyond climate control on species range: the importance of soil data to predict distribution of Amazonian plant species. Journal of Biogeography. 2017;45(1):190–200. doi: 10.1111/jbi.13104. [DOI] [Google Scholar]
- Fine (2015).Fine PVA. Ecological and evolutionary drivers of geographic variation in species diversity. Annual Review of Ecology, Evolution, and Systematics. 2015;46(1):369–392. doi: 10.1146/annurev-ecolsys-112414-054102. [DOI] [Google Scholar]
- Fine & Ree (2006).Fine PVA, Ree RH. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. American Naturalist. 2006;168(6):796–804. doi: 10.1086/508635. [DOI] [PubMed] [Google Scholar]
- Flantua et al. (2015).Flantua SGA, Hooghiemstra H, Van Boxel JH, Cabrera M, Gonzlez-Carranze Z, Gonzlez-Arrango C. Connectivity dynamics since the last glacial maximum in the northern Andes: a pollen-driven framework to assess potential migration. In: Stevens WD, Montiel OMR, Raven PH, editors. Paleobotany and Biogeography; A Festschrift for Alan Graham in His 80 Year. St. Louis: Missouri Botanical Garden Press; 2015. pp. 98–123. [Google Scholar]
- Fleischner et al. (2017).Fleischner TL, Espinoza RE, Gerrish GA, Greene HW, Kimmerer RW, Lacey EA, Pace S, Parrish JK, Swain HM, Trombulak SC, Weisberg S, Winkler DW, Zander L. Teaching biology in the field: importance, challenges, and solutions. BioScience. 2017;67(6):558–567. doi: 10.1093/biosci/bix036. [DOI] [Google Scholar]
- Flynn et al. (1995).Flynn JJ, Wyss AR, Charrier R, Swisher CC. An early Miocene anthropoid skull from the Chilean Andes. Nature. 1995;373(6515):603–607. doi: 10.1038/373603a0. [DOI] [PubMed] [Google Scholar]
- Forest et al. (2007).Forest F, Grenyer R, Rouget M, Davies TJ, Cowling RM, Faith DP, Balmford A, Manning JC, Procheş Ş, van der Bank M, Reeves G, Hedderson TAJ, Savolainen V. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature Communications. 2007;445:757–760. doi: 10.1038/nature05587. [DOI] [PubMed] [Google Scholar]
- Fouquet et al. (2012).Fouquet A, Loebmann D, Castroviejo-Fisher S, Padial JM, Orrico VGD, Lyra ML, Roberto IJ, Kok PJR, Haddad CLFB, Rodrigues MT. From Amazonia to the Atlantic forest: molecular phylogeny of Phyzelaphryninae frogs reveals unexpected diversity and a striking biogeographic pattern emphasizing conservation challenges. Molecular Phylogenetics and Evolution. 2012;65(2):547–561. doi: 10.1016/j.ympev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Fraser et al. (2015).Fraser LH, Pither J, Jentsch A, Sternberg M, Zobel M, Askarizadeh D, Bartha S, Beierkuhnlein C, Bennett JA, Bittel A, Boldgiv B, Boldrini II, Bork E, Brown L, Cabido M, Cahill J, Carlyle CN, Campetella G, Chelli S, Cohen O, Csergo AM, Diaz S, Enrico L, Ensing D, Fidelis A, Fridley JD, Foster B, Garris H, Goheen JR, Henry HAL, Hohn M, Jouri MH, Klironomos J, Koorem K, Lawrence-Lodge R, Long R, Manning P, Mitchell R, Moora M, Muller SC, Nabinger C, Naseri K, Overbeck GE, Palmer TM, Parsons S, Pesek M, Pillar VD, Pringle RM, Roccaforte K, Schmidt A, Shang Z, Stahlmann R, Stotz GC, Sugiyama SI, Szentes S, Thompson D, Tungalag R, Undrakhbold S, Van Rooyen M, Wellstein C, Wilson JB, Zupo T. Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science. 2015;349(6275):302–305. doi: 10.1126/science.aab3916. [DOI] [PubMed] [Google Scholar]
- Fritz et al. (2013).Fritz SA, Schnitzler J, Eronen JT, Hof C, Böhning-Gaese K, Graham CH. Diversity in time and space: wanted dead and alive. Trends in Ecology & Evolution. 2013;28(9):509–516. doi: 10.1016/j.tree.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Fukami (2015).Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annual Review of Ecology, Evolution, and Systematics. 2015;46(1):1–23. doi: 10.1146/annurev-ecolsys-110411-160340. [DOI] [Google Scholar]
- Funk, Caminer & Ron (2011).Funk WC, Caminer M, Ron SR. High levels of cryptic species diversity uncovered in Amazonian frogs. Proceedings of the Royal Society B: Biological Sciences. 2011;279(1734):1806–1814. doi: 10.1098/rspb.2011.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier et al. (2004).Garnier E, Cortez J, Billès G, Navas M-L, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint J-P. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85(9):2630–2637. doi: 10.1890/03-0799. [DOI] [Google Scholar]
- Garreaud & Muñoz (2005).Garreaud RD, Muñoz RC. The low-level jet off the West Coast of Subtropical South America: structure and variability. Monthly Weather Review. 2005;133(8):2246–2261. doi: 10.1175/MWR2972.1. [DOI] [Google Scholar]
- Garzón-Orduña, Benetti Longhini & Brower (2014).Garzón-Orduña IJ, Benetti Longhini JE, Brower AVZ. Timing the diversification of the Amazonian biota: butterfly divergences are consistent with Pleistocene refugia. Journal of Biogeography. 2014;41(9):1631–1638. doi: 10.1111/jbi.12330. [DOI] [Google Scholar]
- Gaston & Spicer (2004).Gaston KJ, Spicer JI. Biodiversity: An Introduction. Oxford: Blackwell Publishing; 2004. [Google Scholar]
- Gehara et al. (2014).Gehara M, Crawford AJ, Orrico VGD, Rodríguez A, Lötters S, Fouquet A, Barrientos LS, Brusquetti F, De La Riva I, Ernst R, Urrutia GG, Glaw F, Guayasamin JM, Hölting M, Jansen M, Kok PJR, Kwet A, Lingnau R, Lyra M, Moravec J, Pombal JP, Rojas-Runjaic FJM, Schulze A, Señaris JC, Solé M, Rodrigues MT, Twomey E, Haddad CFB, Vences M, Köhler J. High levels of diversity uncovered in a widespread nominal taxon: continental phylogeography of the neotropical tree frog Dendropsophus minutus. PLOS ONE. 2014;9(10):e103958. doi: 10.1371/journal.pone.0103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehara et al. (2017).Gehara M, Garda AA, Werneck FP, Oliveira EF, Da Fonseca EM, Camurugi F, Magalhães F De M, Lanna FM, Sites JW, Jr, Marques R, Silveira-Filho R, São Pedro VA, Colli GR, Costa GC, Burbrink FT. Estimating synchronous demographic changes across populations using hABC and its application for a herpetological community from northeastern Brazil. Molecular Ecology. 2017;26(18):4756–4771. doi: 10.1111/mec.14239. [DOI] [PubMed] [Google Scholar]
- Gentry (1982).Gentry AH. Neotropical floristic diversity: phytogeographical connections between central and South America, Pleistocene climatic fluctuations, or an accident of the andean orogeny? Annals of the Missouri Botanical Garden. 1982;69(3):557–593. doi: 10.2307/2399084. [DOI] [Google Scholar]
- Gentry (1988).Gentry AH. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden. 1988;75(1):1–34. doi: 10.2307/2399464. [DOI] [Google Scholar]
- Givnish et al. (2014).Givnish TJ, Barfuss MHJ, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC, Winter K, Brown GK, Evans TM, Holst BK, Luther H, Till W, Zizka G, Berry PE, Sytsma KJ. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution. 2014;71:55–78. doi: 10.1016/j.ympev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Gillman et al. (2014).Gillman LN, Wright SD, Cusens J, McBride PD, Malhi Y, Whittaker RJ. Latitude, productivity and species richness. Global Ecology and Biogeography. 2014;24(1):107–117. doi: 10.1111/geb.12245. [DOI] [Google Scholar]
- Glassman (1999).Glassman SF. A taxonomic treatment of the palm subtribe Attaleinae (Tribe Cocoeae) Urbana: University of Illinois Press; 1999. [Google Scholar]
- Gómez-Acevedo et al. (2010).Gómez-Acevedo S, Rico-Arce L, Delgado-Salinas A, Magallón S, Eguiarte LE. Neotropical mutualism between Acacia and Pseudomyrmex: phylogeny and divergence times. Molecular Phylogenetics and Evolution. 2010;56(1):393–408. doi: 10.1016/j.ympev.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Gotelli & Colwell (2001).Gotelli NJ, Colwell RK. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters. 2001;4(4):379–391. doi: 10.1046/j.1461-0248.2001.00230.x. [DOI] [Google Scholar]
- Gravel et al. (2011).Gravel D, Massol F, Canard E, Mouillot D, Mouquet N. Trophic theory of island biogeography. Ecology Letters. 2011;14(10):1010–1016. doi: 10.1111/j.1461-0248.2011.01667.x. [DOI] [PubMed] [Google Scholar]
- Gray (2004).Gray M. Geodiversity. Chichester: John Wiley & Sons; 2004. [Google Scholar]
- Guedes, Sawaya & Nogueira (2014).Guedes TB, Sawaya RJ, Nogueira C De C. Biogeography, vicariance and conservation of snakes of the neglected and endangered Caatinga region, north-eastern Brazil. Journal of Biogeography. 2014;41(5):919–931. doi: 10.1111/jbi.12272. [DOI] [Google Scholar]
- Gómez et al. (2018).Gómez IC, Sääksjärvi IE, Mayhew PJ, Pollet M, Del Castillo CR, Nieves-Aldrey J-L, Broad GR, Roininen H, Tuomisto H. Variation in the species richness of parasitoid wasps (Ichneumonidae: Pimplinae and Rhyssinae) across sites on different continents. Insect Conservation and Diversity. 2018;11:305–316. [Google Scholar]
- Haffer (1969).Haffer J. Speciation in Amazonian forest birds. Science. 1969;165(3889):131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- Hanson et al. (2012).Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JBH. Beyond biogeographic patterns: processes shaping the microbial landscape. Nature Reviews Microbiology. 2012;10(7):497–506. doi: 10.1038/nrmicro2795. [DOI] [PubMed] [Google Scholar]
- Hausdorf & Hennig (2003).Hausdorf B, Hennig C. Biotic element analysis in biogeography. Systematic Biology. 2003;52(5):717–723. doi: 10.1080/10635150390235584. [DOI] [PubMed] [Google Scholar]
- Hawlitschek, Ramírez Garrido & Glaw (2017).Hawlitschek O, Ramírez Garrido S, Glaw F. How marine currents influenced the widespread natural overseas dispersal of reptiles in the Western Indian Ocean region. Journal of Biogeography. 2017;44(6):1435–1440. doi: 10.1111/jbi.12940. [DOI] [Google Scholar]
- Hazen (1964).Hazen WE. Readings in population and community ecology. The American Midland Naturalist. 1964;72(1):250–253. doi: 10.2307/2423228. [DOI] [Google Scholar]
- Heath, Huelsenbeck & Stadler (2014).Heath TA, Huelsenbeck JP, Stadler T. The fossilized birth-death process for coherent calibration of divergence-time estimates. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):E2957–E2966. doi: 10.1073/pnas.1319091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heithaus (1979).Heithaus ER. Community structure of neotropical flower visiting bees and wasps: diversity and phenology. Ecology. 1979;60(1):190–202. doi: 10.2307/1936480. [DOI] [Google Scholar]
- Henderson, Galeano & Bernal (1997).Henderson A, Galeano A, Bernal R. Field guide to the palms of the Americas. Princeton: Princeton Press University; 1997. [Google Scholar]
- Henderson, Galeano & Bernal (1995).Henderson A, Galeano G, Bernal R. Field guide to the palms of the Americas. Princeton: Princeton University Press; 1995. p. 352. [Google Scholar]
- Hickerson, Stahl & Lessios (2006).Hickerson MJ, Stahl EA, Lessios HA. Test for simultaneous divergence using approximate Bayesian computation. Evolution. 2006;60(12):2435–2453. doi: 10.1554/05-578.1. [DOI] [PubMed] [Google Scholar]
- Hill (1973).Hill M. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54(2):427–432. doi: 10.2307/1934352. [DOI] [Google Scholar]
- Higgins et al. (2011).Higgins MA, Ruokolainen K, Tuomisto H, Llerena N, Cardenas G, Phillips OL, Vásquez R, Räsänen M. Geological control of floristic composition in Amazonian forests. Journal of Biogeography. 2011;38(11):2136–2149. doi: 10.1111/j.1365-2699.2011.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt et al. (2013).Holt BG, Lessard J-P, Borregaard MK, Fritz SA, Araújo MB, Dimitrov D, Fabre P-H, Graham CH, Graves GR, Jønsson KA, Nogués-Bravo D, Wang Z, Whittaker RJ, Fjeldså J, Rahbek C. An update of Wallace’s zoogeographic regions of the world. Science. 2013;339(6115):74–78. doi: 10.1126/science.1228282. [DOI] [PubMed] [Google Scholar]
- Hoorn et al. (2017).Hoorn C, Bogota-A GR, Romero-Baez M, Lammertsma EI, Flantua SGA, Dantas EL, Dino R, Do Carmo DA, Chemale F., Jr The Amazon at sea: onset and stages of the Amazon River from a marine record, with special reference to Neogene plant turnover in the drainage basin. Global and Planetary Change. 2017;153:51–65. doi: 10.1016/j.gloplacha.2017.02.005. [DOI] [Google Scholar]
- Hoorn et al. (1995).Hoorn C, Guerrero J, Sarmiento GA, Lorente MA. Andean tectonics as a cause for changing drainage patterns in Miocene northern South America. Geology. 1995;23(3):237. doi: 10.1130/0091-7613(1995)023<0237:ATAACF>2.3.CO;2. [DOI] [Google Scholar]
- Hoorn, Perrigo & Antonelli (2018).Hoorn C, Perrigo A, Antonelli A. Mountains, climate and biodiversity. New York: Wiley-Blackwell; 2018. [Google Scholar]
- Hoorn et al. (2010a).Hoorn C, Wesselingh FP, Hovikoski J, Guerrero J. The development of the Amazonian mega-wetland (Miocene; Brazil, Colombia, Peru, Bolivia) In: Hoorn C, Wesselingh FP, editors. Amazonia: Landscape and Species Evolution. Oxford: Wiley-Blackwell Publishing Ltd.; 2010a. pp. 123–142. [Google Scholar]
- Hoorn et al. (2010b).Hoorn C, Wesselingh FP, Ter Steege H, Bermudez MA, Mora A, Sevink J, Sanmartín I, Sanchez-Meseguer A, Anderson CL, Figueiredo JP, Jaramillo CA, Riff D, Negri FR, Hooghiemstra H, Lundberg JG, Stadler T, Sarkinen T, Antonelli A. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010b;330(6006):927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- Hoorn et al. (2011).Hoorn C, Wesselingh FP, Ter Steege H, Bermudez MA, Mora A, Sevink J, Sanmartín I, Sanchez-Meseguer A, Anderson CL, Figueiredo JP, Jaramillo CA, Riff D, Negri FR, Hooghiemstra H, Lundberg JG, Stadler T, Sarkinen T, Antonelli A. Origins of biodiversity—response. Science. 2011;331:399–400. doi: 10.1126/science.331.6016.399. [DOI] [PubMed] [Google Scholar]
- Hopkins (2005).Hopkins MJG. Flora da Reserva Ducke, Amazonas, Brasil. Rodriguésia. 2005;56(86):9–25. doi: 10.1590/2175-78602005568602. [DOI] [Google Scholar]
- Horton (2018).Horton BK. Sedimentary record of Andean mountain building. Earth-Science Reviews. 2018;178:279–309. doi: 10.1016/j.earscirev.2017.11.025. [DOI] [Google Scholar]
- Hovenkamp (1997).Hovenkamp P. Vicariance events, not areas, should be used in biogeographical analysis. Cladistics. 1997;13(1–2):67–79. doi: 10.1111/j.1096-0031.1997.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Hrbek et al. (2014).Hrbek T, Da Silva VMF, Dutra N, Gravena W, Martin AR, Farias IP. A new species of river Dolphin from Brazil or: how little do we know our biodiversity. PLOS ONE. 2014;9(1):e83623. doi: 10.1371/journal.pone.0083623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrbek, Seckinger & Meyer (2007).Hrbek T, Seckinger J, Meyer A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Molecular Phylogenetics and Evolution. 2007;43(3):986–998. doi: 10.1016/j.ympev.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hubbell (2001).Hubbell SP. The unified neutral theory of biodiversity and biogeography (MPB-32) Princeton: Princeton University Press; 2001. [Google Scholar]
- Hughes & Eastwood (2006).Hughes CE, Eastwood R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, Pennington & Antonelli (2013).Hughes CE, Pennington RT, Antonelli A. Neotropical plant evolution: assembling the big picture. Botanical Journal of the Linnean Society. 2013;171(1):1–18. doi: 10.1111/boj.12006. [DOI] [Google Scholar]
- Humboldt & Bonpland (1805).Humboldt AV, Bonpland A. Essai sur la géographie des plantes: accompagné d”un tableau physique des régions équinoxiales, fondé sur des mesures exécutées, depuis le dixième degré de latitude boréale jusqu”au dixième degré de latitude australe, pendant les années 1799, 1800, 1801, 1802 et 1803. Paris: Chez Levrault, Schoell et compagnie, libraires; 1805. [Google Scholar]
- Humphreys & Barraclough (2014).Humphreys AM, Barraclough TG. The evolutionary reality of higher taxa in mammals. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1783):20132750. doi: 10.1098/rspb.2013.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries & Parenti (1999).Humphries CJ, Parenti LR. Cladistic biogeography. New York: Oxford University Press; 1999. [Google Scholar]
- Insel, Poulsen & Ehlers (2010).Insel N, Poulsen CJ, Ehlers TA. Influence of the Andes Mountains on South American moisture transport, convection, and precipitation. Climate Dynamics. 2010;35(7–8):1477–1492. doi: 10.1007/s00382-009-0637-1. [DOI] [Google Scholar]
- Jablonski, Roy & Valentine (2006).Jablonski D, Roy K, Valentine JW. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314(5796):102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]
- Jaramillo (2006).Jaramillo CA. Cenozoic plant diversity in the neotropics. Science. 2006;311(5769):1893–1896. doi: 10.1126/science.1121380. [DOI] [PubMed] [Google Scholar]
- Jaramillo et al. (2010).Jaramillo CA, Ochoa D, Contreras L, Pagani M, Carvajal-Ortiz H, Pratt LM, Krishnan S, Cardona A, Romero M, Quiroz L, Rodriguez G, Rueda MJ, La Parra De F, Morón S, Green W, Bayona G, Montes C, Quintero O, Ramirez R, Mora G, Schouten S, Bermudez H, Navarrete R, Parra F, Alvarán M, Osorno J, Crowley JL, Valencia V, Vervoort J. Effects of rapid global warming at the paleocene-eocene boundary on neotropical vegetation. Science. 2010;330(6006):957–961. doi: 10.1126/science.1193833. [DOI] [PubMed] [Google Scholar]
- Jaramillo et al. (2017).Jaramillo CA, Romero I, D’Apolito C, Bayona G, Duarte E, Louwye S, Escobar J, Luque J, Carrillo-Briceño JD, Zapata V, Mora A, Schouten S, Zavada M, Harrington G, Ortiz J, Wesselingh FP. Miocene flooding events of western Amazonia. Science Advances. 2017;3(5):e1601693. doi: 10.1126/sciadv.1601693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins et al. (2015).Jenkins CN, Alves MAS, Uezu A, Vale MM. Patterns of vertebrate diversity and protection in Brazil. PLOS ONE. 2015;10(12):e0145064. doi: 10.1371/journal.pone.0145064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalko & Handley (2001).Kalko EKV, Handley CO. Neotropical bats in the canopy: diversity, community structure, and implications for conservation. Plant Ecology. 2001;153(1–2):319–333. doi: 10.1023/A:1017590007861. [DOI] [Google Scholar]
- Jin & Chakraborty (1994).Jin L, Chakraborty R. Estimation of genetic distance and coefficient of gene diversity from single-probe multilocus DNA fingerprinting data. Molecular Biology and Evolution. 1994;11(1):120–127. doi: 10.1093/oxfordjournals.molbev.a040086. [DOI] [PubMed] [Google Scholar]
- Jost (2006).Jost L. Entropy and diversity. Oikos. 2006;113:363–375. doi: 10.1111/j.2006.0030-1299.14714.x. [DOI] [Google Scholar]
- Kier et al. (2005).Kier G, Mutke J, Dinerstein E, Ricketts TH, Küper W, Kreft H, Barthlott W. Global patterns of plant diversity and floristic knowledge. Journal of Biogeography. 2005;32(7):1107–1116. doi: 10.1111/j.1365-2699.2005.01272.x. [DOI] [Google Scholar]
- Kluge, Bach & Kessler (2008).Kluge J, Bach K, Kessler M. Elevational distribution and zonation of tropical pteridophyte assemblages in Costa Rica. Basic and Applied Ecology. 2008;9(1):35–43. doi: 10.1016/j.baae.2006.11.004. [DOI] [Google Scholar]
- Kluge, Kessler & Dunn (2006).Kluge J, Kessler M, Dunn RR. What drives elevational patterns of diversity? A test of geometric constraints, climate and species pool effects for pteridophytes on an elevational gradient in Costa Rica. Global Ecology and Biogeography. 2006;15(4):358–371. doi: 10.1111/j.1466-822X.2006.00223.x. [DOI] [Google Scholar]
- Kraft & Ackerly (2014).Kraft NJB, Ackerly DD. Assembly of plant communities. In: Monson RK, editor. Ecology and the Environment. New York: Springer; 2014. pp. 67–88. [Google Scholar]
- Kristiansen et al. (2012).Kristiansen T, Svenning JC, Eiserhardt WL, Pedersen D, Brix H, Munch Kristiansen S, Knadel M, Grández C, Balslev H. Environment versus dispersal in the assembly of western Amazonian palm communities. Journal of Biogeography. 2012;39(7):1318–1332. doi: 10.1111/j.1365-2699.2012.02689.x. [DOI] [Google Scholar]
- Kursar et al. (2009).Kursar TA, Dexter KG, Lokvam J, Pennington RT, Richardson JE, Weber MG, Murakami ET, Drake C, McGregor R, Coley PD. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(43):18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagomarsino et al. (2016).Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CA. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae) New Phytologist. 2016;210(4):1430–1442. doi: 10.1111/nph.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis (2017).Landis MJ. Biogeographic dating of speciation times using paleogeographically informed processes. Systematic Biology. 2017;66(2):128–144. doi: 10.1093/sysbio/syw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis et al. (2013).Landis MJ, Matzke NJ, Moore BR, Huelsenbeck JP. Bayesian analysis of biogeography when the number of areas is large. Systematic Biology. 2013;62(6):789–804. doi: 10.1093/sysbio/syt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo & Colli (2017).Ledo RMD, Colli GR. The historical connections between the Amazon and the Atlantic Forest revisited. Journal of Biogeography. 2017;44(11):2551–2563. doi: 10.1111/jbi.13049. [DOI] [Google Scholar]
- Leibold et al. (2004).Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters. 2004;7(7):601–613. doi: 10.1111/j.1461-0248.2004.00608.x. [DOI] [Google Scholar]
- Leite & Rogers (2013).Leite RN, Rogers DS. Revisiting Amazonian phylogeography: insights into diversification hypotheses and novel perspectives. Organisms Diversity & Evolution. 2013;13(4):639–664. doi: 10.1007/s13127-013-0140-8. [DOI] [Google Scholar]
- Lehtonen et al. (2015).Lehtonen S, Jones MM, Zuquim G, Prado J, Tuomisto H. Phylogenetic relatedness within Neotropical fern communities increases with soil fertility. Global Ecology and Biogeography. 2015;24:695–705. [Google Scholar]
- Lemey et al. (2009).Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds its roots. PLOS Computational Biology. 2009;5(9):e1000520. doi: 10.1371/journal.pcbi.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemey et al. (2010).Lemey P, Rambaut A, Welch JJ, Suchard MA. Phylogeography takes a relaxed random walk in continuous space and time. Molecular Biology and Evolution. 2010;27(8):1877–1885. doi: 10.1093/molbev/msq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon & Lemmon (2008).Lemmon A, Lemmon EM. A likelihood framework for estimating phylogeographic history on a continuous landscape. Systematic Biology. 2008;57(4):544–561. doi: 10.1080/10635150802304761. [DOI] [PubMed] [Google Scholar]
- Leprieur et al. (2011).Leprieur F, Tedesco PA, Hugueny B, Beauchard O, Dürr HH, Brosse S, Oberdorff T. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecology Letters. 2011;14(4):325–334. doi: 10.1111/j.1461-0248.2011.01589.x. [DOI] [PubMed] [Google Scholar]
- Levis et al. (2017).Levis C, Costa FRC, Bongers F, Peña-Claros M, Clement CR, Junqueira AB, Neves EG, Tamanaha EK, Figueiredo FOG, Salomão RP, Castilho CV, Magnusson WE, Phillips OL, Guevara JE, Sabatier D, Molino JF, López DC, Mendoza AM, Pitman NCA, Duque A, Vargas PN, Zartman CE, Vasquez R, Andrade A, Camargo JL, Feldpausch TR, Laurance SGW, Laurance WF, Killeen TJ, Nascimento HEM, Montero JC, Mostacedo B, Amaral IL, Vieira ICG, Brienen R, Castellanos H, Terborgh J, De Jesus Veiga Carim M, Da Silva Guimarães JR, De Souza Coelho L, De Almeida Matos FD, Wittmann F, Mogollón HF, Damasco G, Dávila N, García-Villacorta R, Coronado ENH, Emilio T, De Andrade Lima Filho D, Schietti J, Souza P, Targhetta N, Comiskey JA, Marimon BS, Marimon BH, Neill D, Alonso A, Arroyo L, Carvalho FA, De Souza FC, Dallmeier F, Pansonato MP, Duivenvoorden JF, Fine PVA, Stevenson PR, Araujo-Murakami A, C GAA, Baraloto C, Do Amaral DD, Engel J, Henkel TW, Maas P, Petronelli P, Revilla JDC, Stropp J, Daly D, Gribel R, Paredes MR, Silveira M, Thomas-Caesar R, Baker TR, Da Silva NF, Ferreira LV, Peres CA, Silman MR, Cerón C, Valverde FC, Di Fiore A, Jimenez EM, Mora MCP, Toledo M, Barbosa EM, De Matos Bonates LC, Arboleda NC, De Sousa Farias E, Fuentes A, Guillaumet JL, Jørgensen PM, Malhi Y, De Andrade Miranda IP, Phillips JF, Prieto A, Rudas A, Ruschel AR, Silva N, Hildebrand Von P, Vos VA, Zent EL, Zent S, Cintra BBL, Nascimento MT, Oliveira AA, Ramirez-Angulo H, Ramos JF, Rivas G, Schöngart J, Sierra R, Tirado M, Van der Heijden G, Torre EV, Wang O, Young KR, Baider C, Cano A, Farfan-Rios W, Ferreira C, Hoffman B, Mendoza C, Mesones I, Torres-Lezama A, Medina MNU, Van Andel TR, Villarroel D, Zagt R, Alexiades MN, Balslev H, Garcia-Cabrera K, Gonzales T, Hernandez L, Huamantupa-Chuquimaco I, Manzatto AG, Milliken W, Cuenca WP, Pansini S, Pauletto D, Arevalo FR, Reis NFC, Sampaio AF, Giraldo LEU, Sandoval EHV, Gamarra LV, Vela CIA, Ter Steege H. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science. 2017;355(6328):925–931. doi: 10.1126/science.aal0157. [DOI] [PubMed] [Google Scholar]
- Levis et al. (2018).Levis C, Flores BM, Moreira PA, Luize BG, Alves RP, Franco-Moraes J, Lins J, Konings E, Peña Claros M, Bongers F, Costa FRC, Clement CR. How people domesticated Amazonian forests. Frontiers in Ecology and Evolution. 2018;5:15. doi: 10.3389/fevo.2017.00171. [DOI] [Google Scholar]
- Lohmann & Taylor (2014).Lohmann LG, Taylor CM. A new generic classification of tribe Bignonieae (Bignoniaceae)1. Annals of the Missouri Botanical Garden. 2014;99(3):348–489. doi: 10.3417/2003187. [DOI] [Google Scholar]
- Lomolino, Riddle & Whitakker (2017).Lomolino MV, Riddle B, Whitakker RJ. Biogeography. Fourth Edition. Sunderland: Sinauer Associates; 2017. [Google Scholar]
- Losos & Ricklefs (2009).Losos JB, Ricklefs RE. The theory of island biogeography revisited. Princeton: Princeton University Press; 2009. [Google Scholar]
- Lovejoy et al. (2010).Lovejoy NR, Lester K, Crampton WGR, Marques FPL, Albert JS. Phylogeny, biogeography, and electric signal evolution of Neotropical knifefishes of the genus Gymnotus (Osteichthyes: Gymnotidae) Molecular Phylogenetics and Evolution. 2010;54(1):278–290. doi: 10.1016/j.ympev.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Lundberg et al. (2000).Lundberg JG, Kottelat M, Smith GR, Stiassny MLJ, Gill AC. So many fishes, so little time: an overview of recent ichthyological discovery in continental waters. Annals of the Missouri Botanical Garden. 2000;87(1):26–62. doi: 10.2307/2666207. [DOI] [Google Scholar]
- MacArthur & Wilson (1967).MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton: Princeton University Press; 1967. [Google Scholar]
- Magurran (2013).Magurran AE. Measuring Biological Diversity. Oxford: John Wiley & Sons; 2013. [Google Scholar]
- Mahé et al. (2017).Mahé F, De Vargas C, Bass D, Czech L, Stamatakis A, Lara E, Singer D, Mayor J, Bunge J, Sernaker S, Siemensmeyer T, Trautmann I, Romac S, Berney C, Kozlov A, Mitchell EAD, Seppey CVW, Egge E, Lentendu G, Wirth R, Trueba G, Dunthorn M. Parasites dominate hyperdiverse soil protist communities in Neotropical rainforests. Nature Ecology & Evolution. 2017;1:0091. doi: 10.1038/s41559-017-0091. [DOI] [PubMed] [Google Scholar]
- Maldonado et al. (2015).Maldonado C, Molina CI, Zizka A, Persson C, Taylor CM, Albán J, Chilquillo E, Ronsted N, Antonelli A. Estimating species diversity and distribution in the era of Big Data: to what extent can we trust public databases? Global Ecology and Biogeography. 2015;24(8):973–984. doi: 10.1111/geb.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceau, Lambert & Morlon (2015).Manceau M, Lambert A, Morlon H. Phylogenies support out-of-equilibrium models of biodiversity. Ecology Letters. 2015;18(4):347–356. doi: 10.1111/ele.12415. [DOI] [PubMed] [Google Scholar]
- Mao et al. (2012).Mao K, Milne RI, Zhang L, Peng Y, Liu J, Thomas P, Mill RR, Renner SS. Distribution of living Cupressaceae reflects the breakup of Pangea. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):7793–7798. doi: 10.1073/pnas.1114319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalef (1963).Margalef R. On certain unifying principles in ecology. American Naturalist. 1963;97(897):357–374. doi: 10.1086/282286. [DOI] [Google Scholar]
- Marshall & Liebherr (2000).Marshall CJ, Liebherr JK. Cladistic biogeography of the Mexican transition zone. Journal of Biogeography. 2000;27(1):203–216. doi: 10.1046/j.1365-2699.2000.00388.x. [DOI] [Google Scholar]
- Matos-Maraví et al. (2014).Matos-Maraví P, Núñez Aguila R, Peña C, Miller JY, Sourakov A, Wahlberg N. Causes of endemic radiation in the Caribbean: evidence from the historical biogeography and diversification of the butterfly genus Calisto (Nymphalidae: Satyrinae: Satyrini) BMC Evolutionary Biology. 2014;14:199. doi: 10.1186/s12862-014-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos-Maraví (2016).Matos-Maraví P. Investigating the timing of origin and evolutionary processes shaping regional species diversity: insights from simulated data and neotropical butterfly diversification rates. Evolution. 2016;70(7):1638–1650. doi: 10.1111/evo.12960. [DOI] [PubMed] [Google Scholar]
- Matzke (2014).Matzke NJ. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in Island clades. Systematic Biology. 2014;63(6):951–970. doi: 10.1093/sysbio/syu056. [DOI] [PubMed] [Google Scholar]
- McGill et al. (2006).McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution. 2006;21(4):178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- McKenna & Farrell (2006).McKenna DD, Farrell BD. Tropical forests are both evolutionary cradles and museums of leaf beetle diversity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(29):10947–10951. doi: 10.1073/pnas.0602712103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae et al. (2008).McRae BH, Dickson BG, Keitt TH, Shah VB. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology. 2008;89(10):2712–2724. doi: 10.1890/07-1861.1. [DOI] [PubMed] [Google Scholar]
- Melo et al. (2018).Melo WA, Freitas CG, Bacon CD, Collevatti RG. The road to evolutionary success: insights from the demographic history of an Amazonian palm. Heredity. 2018;121(2):183–195. doi: 10.1038/s41437-018-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes et al. (2015).Mendes LW, Tsai SM, Navarrete AA, De Hollander M, Van Veen JA, Kuramae EE. Soil-Borne Microbiome: linking diversity to function. Microbial Ecology. 2015;70(1):255–265. doi: 10.1007/s00248-014-0559-2. [DOI] [PubMed] [Google Scholar]
- Meseguer & Condamine (2018).Meseguer AS, Condamine FL. Ancient tropical extinctions contributed to the latitudinal diversity gradient. bioRxiv. 2018:236646. doi: 10.1101/236646. [DOI] [PubMed] [Google Scholar]
- Meseguer et al. (2015).Meseguer AS, Lobo JM, Ree R, Beerling DJ, Sanmartín I. Integrating fossils, phylogenies, and niche models into biogeography to reveal ancient evolutionary history: the case of Hypericum (Hypericaceae) Systematic Biology. 2015;64(2):215–232. doi: 10.1093/sysbio/syu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer et al. (2015).Meyer C, Kreft H, Guralnick R, Jetz W. Global priorities for an effective information basis of biodiversity distributions. Nature Communications. 2015;6(1):8221. doi: 10.1038/ncomms9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoarivelo et al. (2014).Minoarivelo HO, Hui C, Terblanche JS, Kosakovsky Pond SL, Scheffler K. Detecting phylogenetic signal in mutualistic interaction networks using a Markov process model. Oikos. 2014;123(10):1250–1260. doi: 10.1111/oik.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbach & Schemske (2015).Mittelbach GG, Schemske DW. Ecological and evolutionary perspectives on community assembly. Trends in Ecology and Evolution. 2015;30(5):241–247. doi: 10.1016/j.tree.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Moritz et al. (2000).Moritz C, Patton JL, Schneider CJ, Smith TB. Diversification of rainforest faunas: an integrated molecular approach. Annual Review of Ecology and Systematics. 2000;31(1):533–563. doi: 10.1146/annurev.ecolsys.31.1.533. [DOI] [Google Scholar]
- Morrone (2013).Morrone JJ. Cladistic biogeography of the neotropical region: identifying the main events in the diversification of the terrestrial biota. Cladistics. 2013;30(2):202–214. doi: 10.1111/cla.12039. [DOI] [PubMed] [Google Scholar]
- Moura et al. (2016).Moura MR, Villalobos F, Costa GC, Garcia PCA. Disentangling the role of climate, topography and vegetation in species richness gradients. PLOS ONE. 2016;11(3):e0152468. doi: 10.1371/journal.pone.0152468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento et al. (2013).Nascimento FF, Lazar A, Menezes AN, Da Matta Durans A, Moreira JC, Salazar-Bravo J, Andrea PSD, Bonvicino CR. The role of historical barriers in the diversification processes in open vegetation formations during the Miocene/Pliocene using an ancient rodent lineage as a model. PLOS ONE. 2013;8(4):e61924. doi: 10.1371/journal.pone.0061924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson & Platnick (1980).Nelson G, Platnick NI. A vicariance approach to historical biogeography. BioScience. 1980;30(5):339–343. doi: 10.2307/1307856. [DOI] [Google Scholar]
- Noguera-Urbano & Escalante (2015).Noguera-Urbano EA, Escalante T. Areas de endemismo de los mamíferos (mammalia) neotropicales. Acta Biológica Colombiana. 2015;20(3):47–65. doi: 10.15446/abc.v20n3.46179. [DOI] [Google Scholar]
- Nores (2002).Nores M. An alternative hypothesis for the origin of Amazonian bird diversity. Journal of Biogeography. 2002;26(3):475–485. doi: 10.1046/j.1365-2699.1999.t01-1-00311.x. [DOI] [Google Scholar]
- Novo et al. (2017).Novo NM, Tejedor MF, Pérez ME, Krause JM. New primate locality from the early Miocene of Patagonia, Argentina. American Journal of Physical Anthropology. 2017;164(4):861–867. doi: 10.1002/ajpa.23309. [DOI] [PubMed] [Google Scholar]
- Onstein et al. (2017).Onstein RE, Baker WJ, Couvreur TLP, Faurby S, Svenning JC, Kissling WD. Frugivory-related traits promote speciation of tropical palms. Nature Ecology & Evolution. 2017;1(12):1903–1911. doi: 10.1038/s41559-017-0348-7. [DOI] [PubMed] [Google Scholar]
- Palazzesi et al. (2014).Palazzesi L, Barreda VD, Cuitiño JI, Guler MV, Tellería MC, Santos RV. Fossil pollen records indicate that Patagonian desertification was not solely a consequence of Andean uplift. Nature Communications. 2014;5(1):3558–3579. doi: 10.1038/ncomms4558. [DOI] [PubMed] [Google Scholar]
- Papadopoulou & Knowles (2016).Papadopoulou A, Knowles LL. Toward a paradigm shift in comparative phylogeography driven by trait-based hypotheses. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(29):8018–8024. doi: 10.1073/pnas.1601069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres et al. (2016).Peres CA, Emilio T, Schietti J, Desmoulière SJM, Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(4):892–897. doi: 10.1073/pnas.1516525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escobar et al. (2017).Pérez-Escobar OA, Chomicki G, Condamine FL, Karremans AP, Bogarín D, Matzke NJ, Silvestro D, Antonelli A. Recent origin and rapid speciation of Neotropical orchids in the world’s richest plant biodiversity hotspot. New Phytologist. 2017;215(2):891–905. doi: 10.1111/nph.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret et al. (2007).Perret M, Chautems A, Spichiger R, Barraclough TG, Savolainen V. The geographical pattern of speciation and floral diversification in the neotropics: the tribe Sinningieae (Gesneriaceae) as a case study. Evolution. 2007;61(7):1641–1660. doi: 10.1111/j.1558-5646.2007.00136.x. [DOI] [PubMed] [Google Scholar]
- Phillips et al. (2003).Phillips OL, Vargas PN, Monteagudo AL, Cruz AP, Zans MEC, Sánchez WG, Yli Halla M, Rose S. Habitat association among Amazonian tree species: a landscape-scale approach. Journal of Ecology. 2003;91(5):757–775. doi: 10.1046/j.1365-2745.2003.00815.x. [DOI] [Google Scholar]
- Pilosof et al. (2017).Pilosof S, Porter MA, Pascual M, Kéfi S. The multilayer nature of ecological networks. Nature Ecology & Evolution. 2017;1(4):0101. doi: 10.1038/s41559-017-0101. [DOI] [PubMed] [Google Scholar]
- Prance (1990).Prance GT. The floristic composition of the forest of Central Amazonian Brazil. In: Gentry AH, editor. Four Neotropical Rain Forests. New Haven: Yale University Press; 1990. pp. 112–140. [Google Scholar]
- Prates et al. (2016a).Prates I, Rivera D, Rodrigues MT, Carnaval AC. A mid-Pleistocene rainforest corridor enabled synchronous invasions of the Atlantic Forest by Amazonian anole lizards. Molecular Ecology. 2016a;25(20):5174–5186. doi: 10.1111/mec.13821. [DOI] [PubMed] [Google Scholar]
- Prates et al. (2016b).Prates I, Xue AT, Brown JL, Alvarado-Serrano DF, Rodrigues MT, Hickerson MJ, Carnaval AC. Inferring responses to climate dynamics from historical demography in neotropical forest lizards. Proceedings of the National Academy of Sciences of the United States of America. 2016b;113(29):7978–7985. doi: 10.1073/pnas.1601063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis & Hector (2000).Purvis A, Hector A. Getting the measure of biodiversity. Nature. 2000;405(6783):212–219. doi: 10.1038/35012221. [DOI] [PubMed] [Google Scholar]
- Quintero et al. (2015).Quintero I, Keil P, Jetz W, Crawford FW. Historical biogeography using species geographical ranges. Systematic Biology. 2015;64(6):1059–1073. doi: 10.1093/sysbio/syv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez et al. (2011).Ramírez SR, Eltz T, Fujiwara MK, Gerlach G, Goldman-Huertas B, Tsutsui ND, Pierce NE. Asynchronous diversification in a specialized plant-pollinator mutualism. Science. 2011;333(6050):1742–1746. doi: 10.1126/science.1209175. [DOI] [PubMed] [Google Scholar]
- Räsänen et al. (1995).Räsänen ME, Linna AM, Santos JCR, Negri FR. Late Miocene tidal deposits in the Amazonian foreland basin. Science. 1995;269:386–390. doi: 10.1126/science.269.5222.386. [DOI] [PubMed] [Google Scholar]
- Räsänen, Salo & Kalliola (1987).Räsänen ME, Salo JS, Kalliola RJ. Fluvial perturbance in the western Amazon basin: regulation by long-term Sub-Andean tectonics. Science. 1987;238(4832):1398–1401. doi: 10.1126/science.238.4832.1398. [DOI] [PubMed] [Google Scholar]
- Raven & Axelrod (1974).Raven PH, Axelrod DI. Angiosperm biogeography and past continental movements. Annals of the Missouri Botanical Garden. 1974;61(3):539. doi: 10.2307/2395021. [DOI] [Google Scholar]
- Ree & Sanmartín (2009).Ree RH, Sanmartín I. Prospects and challenges for parametric models in historical biogeographical inference. Journal of Biogeography. 2009;36(7):1211–1220. doi: 10.1111/j.1365-2699.2008.02068.x. [DOI] [Google Scholar]
- Ree & Sanmartín (2018).Ree RH, Sanmartín I. Conceptual and statistical problems with the DEC+J model of founder-event speciation and its comparison with DEC via model selection. Journal of Biogeography. 2018;45(4):741–749. doi: 10.1111/jbi.13173. [DOI] [Google Scholar]
- Ree & Smith (2008).Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology. 2008;57(1):4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- Reis et al. (2016).Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. Journal of Fish Biology. 2016;89(1):12–47. doi: 10.1111/jfb.13016. [DOI] [PubMed] [Google Scholar]
- Rezende, Jordano & Bascompte (2007).Rezende EL, Jordano P, Bascompte J. Effects of phenotypic complementarity and phylogeny on the nested structure of mutualistic networks. Oikos. 2007;116(11):1919–1929. doi: 10.1111/j.2007.0030-1299.16029.x. [DOI] [Google Scholar]
- Ribas et al. (2012).Ribas CC, Aleixo A, Nogueira ACR, Miyaki CY, Cracraft J. A palaeobiogeographic model for biotic diversification within Amazonia over the past three million years. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1729):681–689. doi: 10.1098/rspb.2011.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro et al. (2015).Ribeiro LF, Bornschein MR, Belmonte-Lopes R, Firkowski CR, Morato SAA, Pie MR. Seven new microendemic species of Brachycephalus (Anura: Brachycephalidae) from southern Brazil. PeerJ. 2015;3:e1011. doi: 10.7717/peerj.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs (2008).Ricklefs RE. Disintegration of the ecological community. American Naturalist. 2008;172(6):741–750. doi: 10.1086/593002. [DOI] [PubMed] [Google Scholar]
- Ricklefs & Jenkins (2011).Ricklefs RE, Jenkins DG. Biogeography and ecology: towards the integration of two disciplines. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1576):2438–2448. doi: 10.1098/rstb.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie (2009).Ritchie ME. Scale, heterogeneity, and the structure and diversity of ecological communities. Princeton: Princeton University Press; 2009. [Google Scholar]
- Rohrmann et al. (2016).Rohrmann A, Sachse D, Mulch A, Pingel H, Tofelde S, Alonso RN, Strecker MR. Miocene orographic uplift forces rapid hydrological change in the southern central Andes. Scientific Reports. 2016;6(1):35678. doi: 10.1038/srep35678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas et al. (2012).Rojas D, Vale Á, Ferrero V, Navarro L. The role of frugivory in the diversification of bats in the Neotropics. Journal of Biogeography. 2012;39(11):1948–1960. doi: 10.1111/j.1365-2699.2012.02709.x. [DOI] [Google Scholar]
- Roncal (2006).Roncal J. Habitat differentiation of sympatric Geonoma macrostachys (Arecaceae) varieties in Peruvian lowland forests. Journal of Tropical Ecology. 2006;22(4):483–486. doi: 10.1017/S0266467406003270. [DOI] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Klopfstein S, Vilhelmsen L, Schulmeister S, Murray DL, Rasnitsyn AP. A total-evidence approach to dating with fossils, applied to the early radiation of the hymenoptera. Systematic Biology. 2012;61(6):973–999. doi: 10.1093/sysbio/sys058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist & Sanmartín (2011).Ronquist F, Sanmartín I. Phylogenetic methods in biogeography. Annual Review of Ecology, Evolution, and Systematics. 2011;42(1):441–464. doi: 10.1146/annurev-ecolsys-102209-144710. [DOI] [Google Scholar]
- Rosauer & Jetz (2014).Rosauer DF, Jetz W. Phylogenetic endemism in terrestrial mammals. Global Ecology and Biogeography. 2014;24(2):168–179. doi: 10.1111/geb.12237. [DOI] [Google Scholar]
- Rosen (1975).Rosen DE. A vicariance model of caribbean biogeography. Systematic Zoology. 1975;24(4):431–464. doi: 10.2307/2412905. [DOI] [Google Scholar]
- Rosen (1978).Rosen DE. Vicariant patterns and historical explanation in biogeography. Systematic Biology. 1978;27(2):159–188. doi: 10.2307/2412970. [DOI] [Google Scholar]
- Rosenberger et al. (2009).Rosenberger AL, Tejedor MF, Cooke SB, Pekar S. Platyrrhine ecophylogenetics in space and time. In: Garber P, Estrada A, Bicca-Marquez J, Strier K, editors. South American Primates. New York: Springer; 2009. pp. 69–113. [Google Scholar]
- Rosindell & Harmon (2013).Rosindell J, Harmon LJ. A unified model of species immigration, extinction and abundance on islands. Journal of Biogeography. 2013;40(6):1107–1118. doi: 10.1111/jbi.12064. [DOI] [Google Scholar]
- Rossatto (2014).Rossatto DR. Spatial patterns of species richness and phylogenetic diversity of woody plants in the neotropical savannas of Brazil. Brazilian Journal of Botany. 2014;37(3):283–292. doi: 10.1007/s40415-014-0070-5. [DOI] [Google Scholar]
- Sacek (2014).Sacek V. Drainage reversal of the Amazon River due to the coupling of surface and lithospheric processes. Earth and Planetary Science Letters. 2014;401:301–312. doi: 10.1016/j.epsl.2014.06.022. [DOI] [Google Scholar]
- Safi et al. (2011).Safi K, Cianciaruso MV, Loyola RD, Brito D, Armour-Marshall K, Diniz-Filho JAF. Understanding global patterns of mammalian functional and phylogenetic diversity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1577):2536–2544. doi: 10.1098/rstb.2011.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said Gutiérrez-Ortega et al. (2017).Said Gutiérrez-Ortega J, Yamamoto T, Vovides AP, Angel Pérez-Farrera M, Martínez JF, Molina-Freaner F, Watano Y, Kajita T. Aridification as a driver of biodiversity: a case study for the cycad genus Dioon (Zamiaceae) Annals of Botany. 2017;121(1):47–60. doi: 10.1093/aob/mcx123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo et al. (1986).Salo J, Kalliola R, Häkkinen I, Mäkinen Y, Niemelä P, Puhakka M, Coley PD. River dynamics and the diversity of Amazon lowland forest. Nature. 1986;322(6076):254–258. doi: 10.1038/322254a0. [DOI] [Google Scholar]
- Sandom et al. (2014).Sandom C, Faurby S, Sandel B, Svenning JC. Global late quaternary megafauna extinctions linked to humans, not climate change. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1787):20133254. doi: 10.1098/rspb.2013.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanín et al. (2016).Sanín MJ, Kissling WD, Bacon CD, Borchsenius F, Galeano G, Svenning JC, Olivera J, Ramírez R, Trénel P, Pintaud JC. The Neogene rise of the tropical Andes facilitated diversification of wax palms (Ceroxylon: Arecaceae) through geographical colonization and climatic niche separation. Botanical Journal of the Linnean Society. 2016;182(2):303–317. doi: 10.1111/boj.12419. [DOI] [Google Scholar]
- Sanmartín (2016).Sanmartín I. Breaking the chains of parsimony: the development of parametric methods in historical biogeography. In: Barry Cox C, Moore PD, Ladle R, editors. Biogeography: An Ecological and Evolutionary Approach. Chichester: Wiley-Blackwell; 2016. pp. 239–243. [Google Scholar]
- Sanmartín & Meseguer (2016).Sanmartín I, Meseguer AS. Extinction in Phylogenetics and biogeography: from timetrees to patterns of biotic assemblage. Frontiers in Genetics. 2016;7:35. doi: 10.3389/fgene.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín & Ronquist (2004).Sanmartín I, Ronquist F. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Systematic Biology. 2004;53(2):216–243. doi: 10.1080/10635150490423430. [DOI] [PubMed] [Google Scholar]
- Sanmartín, Van Der Mark & Ronquist (2008).Sanmartín I, Van Der Mark P, Ronquist F. Inferring dispersal: a Bayesian approach to phylogeny-based island biogeography, with special reference to the Canary Islands. Journal of Biogeography. 2008;35(3):428–449. doi: 10.1111/j.1365-2699.2008.01885.x. [DOI] [Google Scholar]
- Santos et al. (2009).Santos JC, Coloma LA, Summers K, Caldwell JP, Ree R, Cannatella DC. Amazonian amphibian diversity is primarily derived from late Miocene Andean Lineages. PLOS Biology. 2009;7(3):e1000056. doi: 10.1371/journal.pbio.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler & Carstens (2016).Satler JD, Carstens BC. Phylogeographic concordance factors quantify phylogeographic congruence among co-distributed species in the Sarracenia alata pitcher plant system. Evolution. 2016;70(5):1105–1119. doi: 10.1111/evo.12924. [DOI] [PubMed] [Google Scholar]
- Satler & Carstens (2017).Satler JD, Carstens BC. Do ecological communities disperse across biogeographic barriers as a unit? Molecular Ecology. 2017;26(13):3533–3545. doi: 10.1111/mec.14137. [DOI] [PubMed] [Google Scholar]
- Satler, Zellmer & Carstens (2016).Satler JD, Zellmer AJ, Carstens BC. Biogeographic barriers drive co-diversification within associated eukaryotes of the Sarracenia alata pitcher plant system. PeerJ. 2016;4:e1576. doi: 10.7717/peerj.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter & Ricklefs (1993).Schluter D, Ricklefs RE. Species diversity: an introduction to the problem. In: Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Chicago: University of Chicago Press; 1993. pp. 1–10. [Google Scholar]
- Sexton et al. (2009).Sexton JP, McIntyre PJ, Angert AL, Rice KJ. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics. 2009;40(1):415–436. doi: 10.1146/annurev.ecolsys.110308.120317. [DOI] [Google Scholar]
- Shephard et al. (2010).Shephard GE, Müller RD, Liu L, Gurnis M. Miocene drainage reversal of the Amazon River driven by plate–mantle interaction. Nature Geoscience. 2010;3(12):870–875. doi: 10.1038/ngeo1017. [DOI] [Google Scholar]
- Silvestro et al. (2015).Silvestro D, Kostikova A, Litsios G, Pearman PB, Salamin N. Measurement errors should always be incorporated in phylogenetic comparative analysis. Methods in Ecology and Evolution. 2015;6(3):340–346. doi: 10.1111/2041-210X.12337. [DOI] [Google Scholar]
- Silvestro et al. (2014).Silvestro D, Schnitzler J, Liow LH, Antonelli A, Salamin N. Bayesian estimation of speciation and extinction from incomplete fossil occurrence data. Systematic Biology. 2014;63(3):349–367. doi: 10.1093/sysbio/syu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro et al. (in press).Silvestro D, Tejedor MF, Serrano-Serrano ML, Loiseau O, Rossier V, Rolland J, Zizka A, Höhna A, Antonelli A, Salamin N. Early arrival and climatically-linked geographic expansion of New World monkeys from tiny African ancestors. Systematic Biology. doi: 10.1093/sysbio/syy046. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro et al. (2016).Silvestro D, Zizka A, Bacon CD, Cascales-Miñana B, Salamin N, Antonelli A. Fossil biogeography: a new model to infer dispersal, extinction and sampling from palaeontological data. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1691):20150225. doi: 10.1098/rstb.2015.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões et al. (2014).Simões PI, Stow A, Hödl W, Amézquita A, Farias IP, Lima AP. The value of including intraspecific measures of biodiversity in environmental impact surveys is highlighted by the Amazonian Brilliant-Thighed Frog (Allobates Femoralis) Tropical Conservation Science. 2014;7(4):811–828. doi: 10.1177/194008291400700416. [DOI] [Google Scholar]
- Simon et al. (2009).Simon MF, Grether R, De Queiroz LP, Skema C, Pennington RT, Hughes CE. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20359–20364. doi: 10.1073/pnas.0903410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al. (2014).Smith BT, McCormack JE, Cuervo AM, Hickerson MJ, Aleixo A, Cadena CD, Pérez-Emán J, Burney CW, Xie X, Harvey MG, Faircloth BC, Glenn TC, Derryberry EP, Prejean J, Fields S, Brumfield RT. The drivers of tropical speciation. Nature. 2014;515(7527):406–409. doi: 10.1038/nature13687. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2017).Smith BT, Seeholzer GF, Harvey MG, Cuervo AM, Brumfield RT. A latitudinal phylogeographic diversity gradient in birds. PLOS Biology. 2017;15(4):e2001073. doi: 10.1371/journal.pbio.2001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Auza & Kessler (2008).Soria-Auza RW, Kessler M. The influence of sampling intensity on the perception of the spatial distribution of tropical diversity and endemism: a case study of ferns from Bolivia. Diversity and Distributions. 2008;14(1):123–130. doi: 10.1111/j.1472-4642.2007.00433.x. [DOI] [Google Scholar]
- Stebbins (1974).Stebbins GL. Flowering plants: evolution above the species level. Cambridge: The Belknap Press of Harvard University Press; 1974. [Google Scholar]
- Strecker et al. (2011).Strecker AL, Olden JD, Whittier JB, Paukert CP. Defining conservation priorities for freshwater fishes according to taxonomic, functional, and phylogenetic diversity. Ecological Applications. 2011;21(8):3002–3013. doi: 10.1890/11-0599.1. [DOI] [Google Scholar]
- Stropp, Ter Steege & Malhi (2009).Stropp J, Ter Steege H, Malhi Y, ATDN. RAINFOR Disentangling regional and local tree diversity in the Amazon. Ecography. 2009;32(1):46–54. doi: 10.1111/j.1600-0587.2009.05811.x. [DOI] [Google Scholar]
- Swenson (2011).Swenson NG. The role of evolutionary processes in producing biodiversity patterns, and the interrelationships between taxonomic, functional and phylogenetic biodiversity. American Journal of Botany. 2011;98(3):472–480. doi: 10.3732/ajb.1000289. [DOI] [PubMed] [Google Scholar]
- Szumik et al. (2002).Szumik CA, Cuezzo F, Goloboff PA, Chalup AE. An optimality criterion to determine areas of endemism. Systematic Biology. 2002;51(5):806–816. doi: 10.1080/10635150290102483. [DOI] [PubMed] [Google Scholar]
- Szumik & Goloboff (2004).Szumik CA, Goloboff PA. Areas of endemism: an improved optimality criterion. Systematic Biology. 2004;53(6):968–977. doi: 10.1080/10635150490888859. [DOI] [PubMed] [Google Scholar]
- Tagliacollo et al. (2015a).Tagliacollo VA, Duke-Sylvester SM, Matamoros WA, Chakrabarty P, Albert JS. Coordinated dispersal and pre-isthmian assembly of the Central American Ichthyofauna. Systematic Biology. 2015a;66(2):183–196. doi: 10.1093/sysbio/syv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliacollo et al. (2015b).Tagliacollo VA, Roxo FF, Duke-Sylvester SM, Oliveira C, Albert JS. Biogeographical signature of river capture events in Amazonian lowlands. Journal of Biogeography. 2015b;42(12):2349–2362. doi: 10.1111/jbi.12594. [DOI] [Google Scholar]
- Tedesco et al. (2017).Tedesco PA, Beauchard O, Bigorne R, Blanchet S, Buisson L, Conti L, Cornu J-F, Dias MS, Grenouillet G, Hugueny B, Jézéquel C, Leprieur F, Brosse S, Oberdorff T. A global database on freshwater fish species occurrence in drainage basins. Scientific Data. 2017;4:170141. doi: 10.1038/sdata.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor & Muñoz-Saba (2013).Tejedor MF, Muñoz-Saba Y. La sistemática de los platirrinos y el registro fósil. In: Defter TR, Stenvenson PR, Bueno ML, Guzmán Caro DC, editors. Primates colombianos en peligro de extinción. Bogotá: researchgate.net; 2013. pp. 68–86. [Google Scholar]
- Tejedor et al. (2006).Tejedor MF, Tauber AA, Rosenberger AL, Swisher CC, Palacios ME. New primate genus from the Miocene of Argentina. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5437–5441. doi: 10.1073/pnas.0506126103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Steege et al. (2011).Ter Steege H, Haripersaud PP, Bánki OS, Schieving F. A model of botanical collectors’ behavior in the field: never the same species twice. American Journal of Botany. 2011;98(1):31–37. doi: 10.3732/ajb.1000215. [DOI] [PubMed] [Google Scholar]
- Ter Steege et al. (2006).Ter Steege H, Pitman NCA, Phillips OL, Chave J, Sabatier D, Duque A, Molino J-F, Prévost M-F, Spichiger R, Castellanos H, Von Hildebrand P, Vasquez R. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature. 2006;443(7110):444–447. doi: 10.1038/nature05134. [DOI] [PubMed] [Google Scholar]
- Ter Steege et al. (2013).Ter Steege H, Pitman NCA, Sabatier D, Baraloto C, Salomão RP, Guevara JE, Phillips OL, Castilho CV, Magnusson WE, Molino J-F, Monteagudo A, Núñez Vargas P, Montero JC, Feldpausch TR, Coronado ENH, Killeen TJ, Mostacedo B, Vasquez R, Assis RL, Terborgh J, Wittmann F, Andrade A, Laurance WF, Laurance SGW, Marimon BS, Marimon B-H, Guimarães Vieira IC, Amaral IL, Brienen R, Castellanos H, Cárdenas López D, Duivenvoorden JF, Mogollón HF, Matos FDDA, Dávila N, García-Villacorta R, Stevenson Diaz PR, Costa F, Emilio T, Levis C, Schietti J, Souza P, Alonso A, Dallmeier F, Montoya AJD, Fernandez Piedade MT, Araujo-Murakami A, Arroyo L, Gribel R, Fine PVA, Peres CA, Toledo M, Aymard C GA, Baker TR, Cerón C, Engel J, Henkel TW, Maas P, Petronelli P, Stropp J, Zartman CE, Daly D, Neill D, Silveira M, Paredes MR, Chave J, Lima Filho DDA, Jørgensen PM, Fuentes A, Schöngart J, Cornejo Valverde F, Di Fiore A, Jimenez EM, Peñuela Mora MC, Phillips JF, Rivas G, Van Andel TR, Von Hildebrand P, Hoffman B, Zent EL, Malhi Y, Prieto A, Rudas A, Ruschell AR, Silva N, Vos V, Zent S, Oliveira AA, Schutz AC, Gonzales T, Trindade Nascimento M, Ramirez-Angulo H, Sierra R, Tirado M, Umaña Medina MN, Van Der Heijden G, Vela CIA, Vilanova Torre E, Vriesendorp C, Wang O, Young KR, Baider C, Balslev H, Ferreira C, Mesones I, Torres-Lezama A, Urrego Giraldo LE, Zagt R, Alexiades MN, Hernandez L, Huamantupa-Chuquimaco I, Milliken W, Palacios Cuenca W, Pauletto D, Valderrama Sandoval E, Valenzuela Gamarra L, Dexter KG, Feeley K, Lopez-Gonzalez G, Silman MR. Hyperdominance in the Amazonian tree flora. Science. 2013;342(6156):1243092. doi: 10.1126/science.1243092. [DOI] [PubMed] [Google Scholar]
- Ter Steege et al. (2016).Ter Steege H, Vaessen RW, Cárdenas López D, Sabatier D, Antonelli A, De Oliveira SM, Pitman NCA, Jørgensen PM, Salomão RP. The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Scientific Reports. 2016;6(1):29549. doi: 10.1038/srep29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz, Malabarba & Knowles (2017).Thomaz AT, Malabarba LR, Knowles LL. Genomic signatures of paleodrainages in a freshwater fish along the southeastern coast of Brazil: genetic structure reflects past riverine properties. Heredity. 2017;119(4):287–294. doi: 10.1038/hdy.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomé et al. (2016).Thomé MTC, Sequeira F, Brusquetti F, Carstens B, Haddad CFB, Rodrigues MT, Alexandrino J. Recurrent connections between Amazon and Atlantic forests shaped diversity in Caatinga four-eyed frogs. Journal of Biogeography. 2016;43(5):1045–1056. doi: 10.1111/jbi.12685. [DOI] [Google Scholar]
- Tilman & Downing (1994).Tilman D, Downing JA. Biodiversity and stability in grasslands. Nature Communications. 1994;367:363–365. doi: 10.1038/367363a0. [DOI] [Google Scholar]
- Toju et al. (2017).Toju H, Yamamichi M, Guimarães PR, Olesen JM, Mougi A, Yoshida T, Thompson JN. Species-rich networks and eco-evolutionary synthesis at the metacommunity level. Nature Ecology & Evolution. 2017;1(2):1–11. doi: 10.1038/s41559-016-0024. [DOI] [PubMed] [Google Scholar]
- Töpel et al. (2016).Töpel M, Zizka A, Calió MF, Scharn R, Silvestro D, Antonelli A. SpeciesGeoCoder: fast categorization of species occurrences for analyses of biodiversity, biogeography, ecology, and evolution. Systematic Biology. 2016;66(2):145–151. doi: 10.1093/sysbio/syw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint et al. (2016).Toussaint A, Charpin N, Brosse S, Villéger S. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Scientific Reports. 2016;6(1):51. doi: 10.1038/srep22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker et al. (2016).Tucker CM, Cadotte MW, Carvalho SB, Davies TJ, Ferrier S, Fritz SA, Grenyer R, Helmus MR, Jin LS, Mooers AO, Pavoine S, Purschke O, Redding DW, Rosauer DF, Winter M, Mazel F. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biological Reviews. 2016;92(2):698–715. doi: 10.1111/brv.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto (2010).Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33(1):2–22. doi: 10.1111/j.1600-0587.2009.05880.x. [DOI] [Google Scholar]
- Tuomisto (2018).Tuomisto H. Different ways of defining diversity, and how to apply them in montane systems. In: Hoorn C, Perrigo A, Antonelli A, editors. Mountains, Climate, and Biodiversity. First Edition. Hoboken: John Wiley & Sons Ltd.; 2018. pp. 295–308. [Google Scholar]
- Tuomisto et al. (2016).Tuomisto H, Moulatlet GM, Balslev H, Emilio T, Figueiredo FOG, Pedersen D, Ruokolainen K. A compositional turnover zone of biogeographical magnitude within lowland Amazonia. Journal of Biogeography. 2016;43(12):2400–2411. doi: 10.1111/jbi.12864. [DOI] [Google Scholar]
- Tuomisto & Poulsen (1996).Tuomisto H, Poulsen AD. Influence of edaphic specialization on pteridophyte distribution in neotropical rain forests. Journal of Biogeography. 1996;23(3):283–293. doi: 10.1046/j.1365-2699.1996.00044.x. [DOI] [Google Scholar]
- Tuomisto, Ruokolainen & Yli-Halla (2003).Tuomisto H, Ruokolainen K, Yli-Halla M. Dispersal, Environment, and Floristic Variation of Western Amazonian Forests. Science. 2003;299:241–244. doi: 10.1126/science.1078037. [DOI] [PubMed] [Google Scholar]
- Tuomisto et al. (2003).Tuomisto H, Poulsen AD, Ruokolainen K, Moran RC, Quintana C, Celi J, Cañas G. Linking floristic patterns with soil heterogeneity and satellite imagery in ecuadorian amazonia. Ecological Applications. 2003;13(2):352–371. doi: 10.1890/1051-0761(2003)013[0352:LFPWSH]2.0.CO;2. [DOI] [Google Scholar]
- Tuomisto et al. (1995).Tuomisto H, Ruokolainen K, Kalliola R, Linna A, Danjoy W, Rodriguez Z. Dissecting Amazonian Biodiversity. Science. 1995;269(5220):63–66. doi: 10.1126/science.269.5220.63. [DOI] [PubMed] [Google Scholar]
- Tuomisto, Zuquim & Cárdenas (2014).Tuomisto H, Zuquim G, Cárdenas G. Species richness and diversity along edaphic and climatic gradients in Amazonia. Ecography. 2014;37(11):1034–1046. doi: 10.1111/ecog.00770. [DOI] [Google Scholar]
- Valdujo, Carnaval & Graham (2013).Valdujo PH, Carnaval ACOQ, Graham CH. Environmental correlates of anuran beta diversity in the Brazilian Cerrado. Ecography. 2013;36(6):708–717. doi: 10.1111/j.1600-0587.2012.07374.x. [DOI] [Google Scholar]
- Valente, Etienne & Phillimore (2014).Valente LM, Etienne RS, Phillimore AB. The effects of island ontogeny on species diversity and phylogeny. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1784):20133227. doi: 10.1098/rspb.2013.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente, Phillimore & Etienne (2015).Valente LM, Phillimore AB, Etienne RS. Equilibrium and non-equilibrium dynamics simultaneously operate in the Galápagos islands. Ecology Letters. 2015;18(8):844–852. doi: 10.1111/ele.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente, Phillimore & Etienne (2018).Valente L, Phillimore AB, Etienne RS. Using molecular phylogenies in island biogeography: it’s about time. Ecography. 2018;182:820. doi: 10.1111/ecog.03503. [DOI] [Google Scholar]
- Van Der Sleen & Albert (2017).Van Der Sleen P, Albert JS. Field Guide to the Fishes of the Amazon, Orinoco, and Guianas. Princeton: Princeton University Press; 2017. [Google Scholar]
- Vellend (2010).Vellend M. Conceptual Synthesis in Community Ecology. The Quarterly Review of Biology. 2010;85(2):183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- Vilhena & Antonelli (2015).Vilhena DA, Antonelli A. A network approach for identifying and delimiting biogeographical regions. Nature Communications. 2015;6(1):1–9. doi: 10.1038/ncomms7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine & Matthews (1963).Vine FJ, Matthews DH. Magnetic anomalies over oceanic ridges. Nature. 1963;199(4897):947–949. doi: 10.1038/199947a0. [DOI] [Google Scholar]
- Wallace (1889).Wallace AR. A narrative of travels on the amazon and rio negro: with an account of the native tribes, and observations on the climate, geology, and natural history of the amazon valley. London: Ward, Lock; 1889. [Google Scholar]
- Wang et al. (2017).Wang X, Edwards RL, Auler AS, Cheng H, Kong X, Wang Y, Cruz FW, Dorale JA, Chiang H-W. Hydroclimate changes across the Amazon lowlands over the past 45,000 years. Nature. 2017;541(7636):204–207. doi: 10.1038/nature20787. [DOI] [PubMed] [Google Scholar]
- Warren et al. (2015).Warren BH, Simberloff D, Ricklefs RE, Aguilée R, Condamine FL, Gravel D, Morlon H, Mouquet N, Rosindell J, Casquet J, Conti E, Cornuault J, Fernández-Palacios JM, Hengl T, Norder SJ, Rijsdijk KF, Sanmartín I, Strasberg D, Triantis KA, Valente LM, Whittaker RJ, Gillespie RG, Emerson BC, Thébaud C. Islands as model systems in ecology and evolution: prospects fifty years after MacArthur-Wilson. Ecology Letters. 2015;18(2):200–217. doi: 10.1111/ele.12398. [DOI] [PubMed] [Google Scholar]
- Webb et al. (2003).Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology and Systematics. 2003;33(1):475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448. [DOI] [Google Scholar]
- Weber & Strauss (2016).Weber MG, Strauss SY. Coexistence in close relatives: beyond competition and reproductive isolation in sister taxa. Annual Review of Ecology, Evolution, and Systematics. 2016;47(1):359–381. doi: 10.1146/annurev-ecolsys-112414-054048. [DOI] [Google Scholar]
- Weeks, Claramunt & Cracraft (2016).Weeks BC, Claramunt S, Cracraft J. Integrating systematics and biogeography to disentangle the roles of history and ecology in biotic assembly. Journal of Biogeography. 2016;43(8):1546–1559. doi: 10.1111/jbi.12747. [DOI] [Google Scholar]
- Wegener (1912).Wegener A. Die Entstehung der Kontinente. Geologische Rundschau. 1912;3(4):276–292. doi: 10.1007/BF02202896. [DOI] [Google Scholar]
- Werneck et al. (2011).Werneck FP, Costa GC, Colli GR, Prado DE, Sites JW., Jr Revisiting the historical distribution of Seasonally Dry Tropical Forests: new insights based on palaeodistribution modelling and palynological evidencegeb. Global Ecology and Biogeography. 2011;20(2):272–288. doi: 10.1111/j.1466-8238.2010.00596.x. [DOI] [Google Scholar]
- Werneck et al. (2012a).Werneck FP, Gamble T, Colli GR, Rodrigues MT, Sites JW., Jr Deep diversification and long-term persistence in the South American “dry diagonal”: integrating continent-wide phylogeography and distribution modeling of geckos. Evolution. 2012a;66(10):3014–3034. doi: 10.1111/j.1558-5646.2012.01682.x. [DOI] [PubMed] [Google Scholar]
- Werneck et al. (2012b).Werneck FP, Nogueira C, Colli GR, Sites JW, Costa GC. Climatic stability in the Brazilian Cerrado: implications for biogeographical connections of South American savannas, species richness and conservation in a biodiversity hotspot. Journal of Biogeography. 2012b;39(9):1695–1706. doi: 10.1111/j.1365-2699.2012.02715.x. [DOI] [Google Scholar]
- Whittaker, Triantis & Ladle (2008).Whittaker RJ, Triantis KA, Ladle RJ. A general dynamic theory of oceanic island biogeography. Journal of Biogeography. 2008;35(6):977–994. doi: 10.1111/j.1365-2699.2008.01892.x. [DOI] [Google Scholar]
- Wiens & Donoghue (2004).Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends in Ecology & Evolution. 2004;19(12):639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Willig, Kaufman & Stevens (2003).Willig MR, Kaufman DM, Stevens RD. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annual Review of Ecology, Evolution, and Systematics. 2003;34(1):273–309. doi: 10.1146/annurev.ecolsys.34.012103.144032. [DOI] [Google Scholar]
- Wilson et al. (2012).Wilson JB, Peet RK, Dengler J, Pärtel M. Plant species richness: the world records. Journal of Vegetation Science. 2012;23(4):796–802. doi: 10.1111/j.1654-1103.2012.01400.x. [DOI] [Google Scholar]
- Wing et al. (2009).Wing SL, Herrera F, Jaramillo CA, Gómez-Navarro C, Wilf P, Labandeira CC. Late Paleocene fossils from the Cerrejon Formation, Colombia, are the earliest record of Neotropical rainforest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18627–18632. doi: 10.1073/pnas.0905130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worm et al. (2006).Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- Zedane et al. (2016).Zedane L, Hong-Wa C, Murienne J, Jeziorski C, Baldwin BG, Besnard G. Museomics illuminate the history of an extinct, paleoendemic plant lineage (Hesperelaea, Oleaceae) known from an 1875 collection from Guadalupe Island, Mexico. Biological Journal of the Linnean Society. 2016;117(1):44–57. doi: 10.1111/bij.12509. [DOI] [Google Scholar]
- Zhang et al. (2016).Zhang C, Stadler T, Klopfstein S, Heath TA, Ronquist F. Total-evidence dating under the fossilized birth–death process. Systematic Biology. 2016;65(2):228–249. doi: 10.1093/sysbio/syv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizka et al. (2017).Zizka A, Ter Steege H, Pessoa MDCR, Antonelli A. Finding needles in the haystack: where to look for rare species in the American tropics. Ecography. 2017;41(2):321–330. doi: 10.1111/ecog.02192. [DOI] [Google Scholar]
- Zuquim et al. (2009).Zuquim G, Costa FRC, Prado J, Braga-Neto R. Distribution of pteridophyte communities along environmental gradients in Central Amazonia, Brazil. Biodiversity and Conservation. 2009;18(1):151–166. doi: 10.1007/s10531-008-9464-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this literature review did not generate any data or code.