Abstract
The purpose was to analyze statin effectiveness in a general population with differing levels of coronary heart disease (CHD) risk. Patients (35–74 years) without previous cardiovascular disease were included and stratified according to 10‐year CHD risk (<5%, 5–7.4%, 7.5–9.9%, and 10–19.9%). New users were categorized according to their medical possession ratio (MPR). The main outcome was atherosclerotic cardiovascular disease (ASCVD) (myocardial infarction and ischemic stroke). In adherent patients (MPR 70%), statin treatment decreased ASCVD risk across the range of coronary risk (from 16–30%). The 5‐year number needed to treat (NNT) was 470 and 204 in the risk categories <5% and 5–7.4%, respectively, and 75 and 62 in the 7.5–9.9% category than in the 10–19.9% category, respectively. Statin therapy should remain a priority in patients at high 10‐year CHD risk (10–19.9%). Most patients with intermediate risk could benefit from statin treatment, but the treatment decision should focus on the net benefit, safety, and patient preference, given the higher NNT.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
☑ The evidence of statin efficiency and safety is well known in primary and secondary prevention. However, statin effectiveness, in terms of absolute benefit, and the cardiovascular risk threshold from which individuals should initiate treatment remain uncertain.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We examined if statins are effective in terms of net benefit across different ranges of 10‐year coronary risk.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ Statins were effective in reducing cardiovascular events across the range of 10‐year coronary risk groups in adherent individuals. The NNT in the lower risk categories (<5% and 5–7.4%) was higher than in the moderate and high‐risk categories (7.5–9.9% and 10–19·9%).
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ Statins should remain a priority at high 10‐year coronary risk (10–19.9%) because of the clear absolute benefit and may be useful in the intermediate category (7.5–9.9%), although the decision to initiate treatment should focus on the absolute benefit, safety, and patient preferences. In addition, a first‐line strategy to improve statins effectiveness in primary care should be the implementation of interventions to improve adherence.
New guidelines to manage dyslipidemia recommend individualized prevention measures to reduce the incidence of cardiovascular events and focus on two benefit groups: first, secondary prevention patients, diabetics, and patients with one or more cardiovascular risk factors (dyslipidemia, hypertension, or smoking) or with chronic kidney disease; and second, individuals with 10‐year cardiovascular risk estimates.1, 2, 3, 4 These guidelines have lowered the estimated 10‐year cardiovascular risk threshold to reduce the incidence of cardiovascular events. In primary prevention, European guidelines recommend treatment in individuals with 5% or higher risk on the SCORE 10‐year fatal cardiovascular risk chart and in those with low (<5%) risk and low‐density lipoprotein (LDL) cholesterol levels between 2.6 and 4 mmol/L.4 US guidelines recommend treatment for individuals aged 40–75 years with 10‐year risk scores 7.5%; each guide, however, with different levels of evidence.1, 2 In the United Kingdom, the National Institute for Health and Care Excellence suggests statin treatment in primary prevention for people with 10% risk.3 The definition of high cardiovascular risk varies across guidelines and depends on the risk function used, the endpoints considered, the reference population, and the presence of comorbidities. These recommendations are based on the results from previous meta‐analyses5, 6 and recent clinical trials,7 which show that the efficacy of statins is similar in high and intermediate cardiovascular risk populations.
These threshold recommendations are controversial for several reasons. First, the population eligible for lifetime statin therapy would increase substantially. Second, the focus is on relative rather than absolute risk reduction.8, 9, 10 Finally, the randomization of the clinical trials upon which the guidelines are based did not take baseline cardiovascular risk into account.
As participants in clinical trials may not represent the general population because specific groups (statin‐intolerant or poorly adherent, diabetics, women, young people) are often excluded,11 data from electronic medical records can be used to provide population‐based evidence for cardiovascular risk management decisions and to evaluate treatment effectiveness in real‐life clinical conditions.12
The aim of the present study was to analyze the effectiveness of statins in a general population according to their coronary risk estimation.
RESULTS
Between July 2006 and December 2007, 617,850 individuals fulfilled all inclusion criteria and 20,799 (3.3%) initiated statin therapy. Losses to follow‐up were 3.1% (n = 19,557), all of them due to transfer out of the Information System for the Development of Research in Primary Care (SIDIAPQ) database. Median follow‐up was 7.7 years (ranging from 7.2, 1st quartile, to 8.0, 3rd quartile). There were 523,580 participants with 10‐year coronary heart disease (CHD) risk <5% (84.7%), 53,534 with risk 5–7.4% (8.6%), 21,824 with risk 7.5–9.9% (3.5%), and 18,912 with risk 10–19.9% (3.0%). The percentage of individuals with complete follow‐up was: 91.05% (CHD category <5%), 84.41% (5–7.4%), 80.27% (7.5–9.9%), and 75.37% (10–19.9%).
The study flowchart is detailed in Figure 1. The missing data for incomplete variables and a comparison of the complete‐case and imputed datasets are shown in Supplementary Table S1. Mean values of these variables were lower after multiple imputations, as expected.
Figure 1.
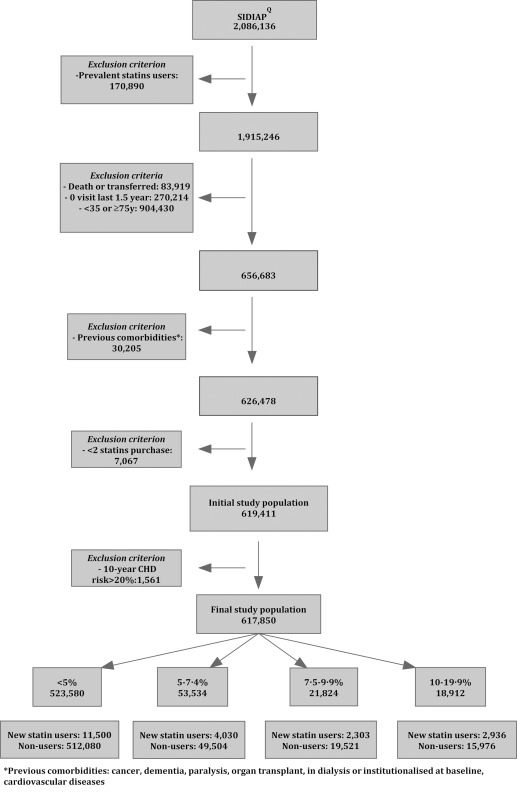
Flow chart of the study population.
Baseline characteristics
Women constituted 53.8% of the sample. Mean age was 50.4 (SD 10.5) years. Diabetes was present in 4.9% of participants, hypertension in 17.38%, smoking in 34.8%, and dyslipidemia in 15.2%. The proportion of adherent new users (6‐month medical possession ratio (MPR) 70%) was 54.9%; median MPR was 76.6% (1st quartile, 46.0; 3rd quartile, 100). Descriptive analysis of the number of days covered by statins is shown in Table S2.
Main baseline characteristics of patients with high (MPR 70%) and low (MPR <70%) statin adherence and of nonusers are shown in Table 1, stratified by CHD risk before propensity score (PS) adjustment. Main baseline characteristics after PS adjustment are shown in Table 2. No clinically relevant standardized differences were observed after adjusting for PS. Other baseline characteristics before and after PS adjustment are shown in Tables S3, S4, respectively. Over 80% of new users were treated with a statin of moderate LDL‐reduction capacity (Table S4). Baseline characteristics after PS adjustment are also shown for the complete dataset in Table S5.
Table 1.
Baseline characteristics of the total population by coronary risk before adjusting for propensity score
| <5% | SDiffa | SDiffa | ≥5%‐7.4% | SDiffa | SDiffa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Statin nonusers | Statin users MPR < 70% | Statin users MPR ≥ 70% | Statin nonusers | Statin users MPR < 70% | Statin users MPR ≥ 70% | ||||
| Age, mean (SD) years | 48.50 (9.75) | 55.23 (9.97) | 57.24 (9.63) | −0.68 | −0.9 | 58.62 (8.46) | 58.09 (8.53) | 59.50 (7.98) | 0.06 | −0.11 |
| Sex (% women) | 59.18 | 71.40 | 74.79 | −0.26 | −0.34 | 26.92 | 41.47 | 49.39 | −0.31 | −0.48 |
| High‐risk alcohol intake (%) | 2.65 | 2.26 | 1.79 | 0.02 | 0.06 | 6.73 | 4.47 | 4.03 | 0.1 | 0.12 |
| Smokers (%) | 31.13 | 23.17 | 19.72 | 0.18 | 0.26 | 51.29 | 39.60 | 32.29 | 0.24 | 0.39 |
| Diabetes (%) | 2.06 | 6.26 | 8.87 | −0.21 | −0.3 | 13.33 | 21.10 | 26.79 | −0.21 | −0.34 |
| Hypertension (%) | 13.08 | 29.09 | 39.00 | −0.4 | −0.62 | 33.47 | 43.15 | 49.44 | −0.2 | −0.33 |
| Dyslipidemia (%) | 11.62 | 57.28 | 72.82 | −1.1 | −1.58 | 23.10 | 60.38 | 71.45 | −0.82 | −1.11 |
| Obesity (%) | 30.04 | 39.55 | 40.71 | −0.2 | −0.22 | 43.49 | 45.88 | 49.27 | −0.05 | −0.12 |
| Chronic kidney disease (%) | 0.22 | 0.79 | 1.04 | −0.08 | −0.1 | 0.64 | 0.84 | 1.13 | −0.02 | −0.05 |
| Blood pressure | ||||||||||
| Systolic, mean (SD), mm Hg | 125.19 (15.06) | 128.00(14.54) | 128.71(14.59) | −0.19 | −0.24 | 138.20(15.67) | 136.94(15.75) | 136.93(16.18) | 0.08 | 0.08 |
| Diastolic, mean (SD), mm Hg | 76.63(9.53) | 77.65(9.06) | 77.63(8.96) | −0.11 | −0.11 | 81.88(10.06) | 81.25(9.74) | 80.84(9.75) | 0.06 | 0.1 |
| Total cholesterol, mean (SD), mmol/L | 5.19(0.88) | 6.54(1.10) | 6.81(1.04) | −1.36 | −1.68 | 5.64(0.92) | 6.62(1.11) | 6.75(1.04) | −0.96 | −1.13 |
| Total cholesterol, mean (SD), mg/dl | 200.79(34.12) | 253.01(42.48) | 263.17(40.08) | −1.36 | −1.68 | 218.06(35.68) | 256.10(43.08) | 260.93(40.06) | −0.96 | −1.13 |
| LDL‐cholesterol,mean (SD), mmol/L | 3.26(0.79) | 4.37(0.97) | 4.55(0.92) | −1.25 | −1.5 | 3.48(0.83) | 4.36(0.99) | 4.48(0.94) | −0.96 | −1.12 |
| LDL cholesterol, mean (SD), mg/dl | 126.02(30.70) | 168.93(37.59) | 175.96(35.60) | −1.25 | −1.50 | 134.39(32.25) | 168.58(38.37) | 173.06(36.48) | −0.96 | −1.13 |
| HDL cholesterol, mean (SD), mmol/L | 1.52(0.40) | 1.58(0.38) | 1.61(0.37) | −0.15 | −0.22 | 1.26(0.30) | 1.32(0.30) | 1.34(0.29) | −0.2 | −0.29 |
| HDL cholesterol, mean (SD), mg/dl | 58.89(15.33) | 61.11(14.58) | 62.10(14.26) | −0.15 | −0.22 | 48.62(11.77) | 51.01(11.73) | 52.00(11.26) | −0.20 | −0.29 |
| Serum triglycerides, mmol/L | 1.21(0.68) | 1.57(1.06) | 1.60(1.09) | −0.41 | −0.43 | 1.52(0.91) | 1.99(1.45) | 1.94(1.19) | −0.39 | −0.40 |
| Serum triglycerides, mg/dl | 106.76(60.28) | 139.41(93.58) | 141.48(96.40) | −0.41 | −0.43 | 134.20(80.26) | 175.89(128.13) | 171.69(105.82) | −0.39 | −0.40 |
| Glucose, mmol/L | 5.08(0.95) | 5.43(1.40) | 5.59(1.61) | −0.29 | −0.39 | 5.76(1.58) | 6.19(2.19) | º6.43(2.25) | −0.23 | −0.34 |
| Glucose, mg/dl | 91.43(17.02) | 97.77(25.26) | 100.57(28.9) | −0.29 | −0.39 | 103.67(28.39) | 111.5(39.5) | 115.67(40.52) | −0.23 | −0.34 |
| Body mass index, kg/m2 | 27.26(5.30) | 28.69(5.31) | 28.85(5.25) | −0.27 | −0.3 | 29.27(5.32) | 29.64(5.36) | 29.92(5.19) | −0.07 | −0.12 |
| 10‐year CHD risk, mean (SD) | 1.97(1.22) | 2.88(1.14) | 3.06(1.09) | −0.77 | −0.95 | 6.04(0.71) | 6.07(0.70) | 6.15(0.72) | −0.05 | −0.15 |
| ≥7.5‐9.9% | SDiffb | SDiffb | ≥10‐19.9% | SDiffa | SDiffa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Statin nonusers | Statin users MPR<70 | Statin users MPR≥70 | Statin nonusers | Statin users MPR<70 | Statin users MPR≥70 | ||||
| Age, mean (SD) years | 61.83(7.71) | 59.73(7.76) | 60.83(7.66) | 0.27 | 0.13 | 65.04(6.74) | 62.45(7.06) | 63.40(6.87) | 0.38 | 0.24 |
| Sex (% women) | 16.26 | 27.43 | 29.17 | −0.27 | −0.31 | 9.32 | 15.60 | 17.17 | −0.19 | −0.23 |
| High‐risk alcohol intake (%) | 8.17 | 7.17 | 6.62 | 0.04 | 0.06 | 8.54 | 10.56 | 7.28 | −0.07 | 0.05 |
| Smokers (%) | 60.65 | 54.40 | 44.16 | 0.13 | 0.33 | 73.00 | 67.03 | 62.06 | 0.13 | 0.24 |
| Diabetes (%) | 21.88 | 31.75 | 37.73 | −0.22 | −0.35 | 31.81 | 43.67 | 50.65 | −0.25 | −0.39 |
| Hypertension (%) | 39.99 | 46.18 | 53.95 | −0.13 | −0.28 | 46.07 | 52.72 | 61.00 | −0.13 | −0.3 |
| Dyslipidemia (%) | 25.24 | 61.84 | 71.00 | −0.79 | −1.03 | 27.62 | 61.98 | 69.81 | −0.74 | −0.93 |
| Obesity, No (%) | 45.14 | 44.89 | 47.85 | 0.01 | −0.05 | 47.25 | 46.11 | 49.60 | 0.02 | −0.05 |
| Chronic kidney disease (%) | 0.92 | 0.97 | 1.01 | −0.01 | −0.01 | 1.22 | 1.76 | 2.02 | −0.04 | −0.06 |
| Blood pressure | ||||||||||
| Systolic, mean (SD), mm Hg | 141.01(15.87) | 139.43(16.40) | 139.48(16.61) | 0.1 | 0.09 | 144.82(15.76) | 143.70(16.24) | 143.69(16.23) | 0.07 | 0.07 |
| Diastolic, mean (SD), mm Hg | 82.31(10.20) | 81.83(10.13) | 81.82(9.80) | 0.05 | 0.05 | 82.66(10.09) | 82.57(10.17) | 82.06(10.09) | 0.01 | 0.06 |
| Total cholesterol, mean (SD), mmol/L | 5.68(0.94) | 6.62(1.10) | 6.66(1.05) | −0.92 | −0.99 | 5.76(0.96) | 6.58(1.00) | 6.61(1.02) | −0.83 | −0.85 |
| Total cholesterol, mean (SD), mg/dl | 219.62(36.45) | 256.04(42.65) | 257.61(40.48) | −0.92 | −0.99 | 222.91(37.15) | 254.63(38.81) | 255.50(39.55) | −0.83 | −0.85 |
| LDL cholesterol, mean (SD), mmol/L | 3.48(0.84) | 4.37(0.97) | 4.39(0.91) | −0.99 | −1.04 | 3.50(0.84) | 4.31(0.93) | 4.33(0.88) | −0.92 | −0.97 |
| LDL cholesterol, mean (SD), mg/dl | 134.53(32.43) | 169.15(37.42) | 169.69(35.27) | −0.99 | −1.04 | 135.31(32.45) | 166.75(35.77) | 167.61(33.90) | −0.92 | −0.97 |
| HDL cholesterol, mean (SD), mmol/L | 1.22(0.29) | 1.25(0.28) | 1.26(0.26) | −0.14 | −0.17 | 1.15(0.26) | 1.18(0.26) | 1.18(0.25) | −0.11 | −0.14 |
| HDL cholesterol, mean (SD), mg/dl | 47.00(11.25) | 48.52(10.93) | 48.84(10.10) | −0.14 | −0.17 | 44.48(9.97) | 45.55(10.10) | 45.81(9.56) | −0.11 | −0.14 |
| Serum triglycerides, mmol/L | 1.58(0.96) | 2.08(1.34) | 2.12(1.48) | −0.42 | −0.43 | 1.66(1.06) | 2.23(1.58) | 2.24(1.62) | −0.42 | −0.42 |
| Serum triglycerides, mg/dl | 140.16(85.39) | 183.85(119.07) | 187.80(131.23) | −0.42 | −0.43 | 147.38(94.12) | 197.83(140.35) | 198.74(143.38) | −0.42 | −0.42 |
| Glucose, mmol/L | 6.12(1.94) | 6.63(2.44) | 6.96(2.7) | −0.23 | −0.36 | 6.54(2.22) | 7.12(2.6) | 7.63(3.19) | −0.24 | −0.40 |
| Glucose, mg/dl | 110.09(34.86 | 119.33(43.95) | 125.21(48.6) | −0.23 | −0.36 | 117.65(40.04) | 128.21(47.12) | 137.41(57.38) | −0.24 | −0.40 |
| Body Mass Index, kg/m2 | 29.48(5.23) | 29.61(5.13) | 29.88(5.03) | −0.02 | −0.08 | 29.67 5.14 | 29.67 4.92 | 29.85 4.81 | 0.00 | −0.04 |
| 10‐year CHD risk, mean (SD) | 8.58(0.70) | 8.62(0.71) | 8.67(0.70) | −0.06 | −0.12 | 12.90(2.44) | 13.18(2.52) | 13.44(2.60) | −0.11 | −0.21 |
SDiff: standardized differences with respect to nonusers.
SDiff: standardized differences with respect to nonusers.
Table 2.
Baseline characteristics of the total population by coronary risk after adjusting for propensity score
| <5% | SDiffa | SDiffa | ≥5‐7.4% | SDiffa | SDiffa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Statin nonusers | Statin users MPR < 70% | Statin users MPR ≥ 70% | Statin nonusers | Statin users MPR<70% | Statin users MPR ≥ 70% | ||||
| Age, mean (SD) years | 48.69(8.94) | 47.65(10.59) | 48.10(9.91) | 0.11 | 0.06 | 58.72(7.19) | 58.04(7.99) | 57.44(7.31) | 0.09 | 0.18 |
| Sex (% women) | 59.57 | 66.25 | 67.56 | −0.14 | −0.17 | 26.39 | 25.08 | 24.45 | 0.03 | 0.04 |
| High‐risk alcohol intake (%) | 2.65 | 2.01 | 1.53 | 0.04 | 0.08 | 6.63 | 4.96 | 4.71 | 0.07 | 0.08 |
| Smokers (%) | 30.83 | 29.34 | 26.22 | 0.03 | 0.1 | 48.56 | 54.03 | 56.99 | −0.11 | −0.17 |
| Diabetes (%) | 1.53 | 1.19 | 1.28 | 0.03 | 0.02 | 11.13 | 9.8 | 8.71 | 0.04 | 0.08 |
| Hypertension (%) | 11.32 | 8.5 | 9.79 | 0.09 | 0.05 | 33.47 | 31.19 | 29.32 | 0.05 | 0.09 |
| Dyslipidemia (%) | 2.34 | 1.3 | 1.31 | 0.08 | 0.08 | 15.17 | 13.01 | 10.86 | 0.06 | 0.13 |
| Obesity (%) | 29.91 | 28.88 | 28.79 | 0.02 | 0.02 | 43.81 | 42.81 | 42.22 | 0.02 | 0.03 |
| Chronic kidney disease (%) | 0.16 | 0.14 | 0.14 | 0.01 | 0.01 | 0.52 | 0.42 | 0.38 | 0.01 | 0.02 |
| Blood pressure | ||||||||||
| Systolic, mean (SD), mm Hg | 125.28(14.83) | 124.30(14.64) | 124.65(14.70) | 0.07 | 0.04 | 138.04(15.62) | 138.55(15.77) | 139.02(16.21) | −0.03 | −0.06 |
| Diastolic, mean (SD), mm Hg | 76.66(9.36) | 76.26(9.09) | 76.35(9.00) | 0.04 | 0.03 | 81.78(9.98) | 82.12(9.80) | 82.39(9.74) | −0.04 | −0.06 |
| Total cholesterol, mean (SD), mmol/L | 5.22(0.60) | 5.25(0.75) | 5.26(0.73) | −0.04 | −0.05 | 5.71(0.67) | 5.77(0.83) | 5.81(0.80) | −0.08 | −0.14 |
| Total cholesterol, mean (SD), mg/dl | 202.02(23.10) | 203.10(29.01) | 203.28(28.21) | −0.04 | −0.04 | 220.88(25.97) | 223.27(32.13) | 224.80(31.08) | −0.08 | −0.13 |
| LDL cholesterol, mean (SD), mmol/L | 3.28(0.60) | 3.35(0.71) | 3.33(0.69) | −0.09 | −0.07 | 3.54(0.63) | 3.60(0.75) | 3.62(0.74) | −0.09 | −0.11 |
| LDL cholesterol, mean (SD), mg/dl | 126.99(23.29) | 129.40(27.60) | 128.81(26.75) | −0.09 | −0.07 | 136.96(24.39) | 139.40(29.01) | 139.86(28.45) | −0.09 | −0.10 |
| HDL cholesterol, mean (SD), mmol/L | 1.52(0.39) | 1.54(0.38) | 1.55(0.37) | −0.04 | −0.06 | 1.26(0.30) | 1.26(0.30) | 1.26(0.29) | 0.01 | 0.02 |
| HDL cholesterol, mean (SD), mg/dl | 58.93(15.21) | 59.53(14.53) | 59.77(14.19) | −0.04 | −0.05 | 48.86(11.55) | 48.78(11.51) | 48.59(11.05) | 0.01 | 0.02 |
| Serum triglycerides, mmol/L | 1.21(0.67) | 1.40(1.05) | 1.39(1.09) | −0.21 | −0.2 | 1.53(0.90) | 1.84(1.44) | 1.78(1.19) | −0.26 | −0.23 |
| Serum triglycerides, mg/dl | 107.14(59.74) | 123.91(93.29) | 123.14(96.31) | −0.21 | −0.19 | 135.33(79.58) | 162.53(127.96) | 157.22(105.57) | −0.26 | −0.23 |
| Glucose, mmol/L | 5.09(0.93) | 5.06 (1.39) | 5.13 (1.6) | 0.02 | −0.03 | 5.8(1.52) | 5.79(2.12) | 5.8(2.21) | 0.00 | 0.00 |
| Glucose, mg/dl | 91.59(16.67) | 91.11(25.00) | 92.35(28.73) | 0.02 | −0.03 | 104.45(27.35) | 104.29(38.07) | 104.35(39.8) | 0.00 | 0.00 |
| Body mass index, kg/m2 | 27.29(5.22) | 27.29(5.36) | 27.3(5.28) | 0.00 | 0.00 | 29.33(5.27) | 29.2(5.31) | 29.13(5.17) | 0.02 | 0.04 |
| 10‐year CHD risk, mean (SD) | 1.99(1.10) | 1.86(1.15) | 1.86(1.11) | 0.12 | 0.12 | 6.04(0.70) | 6.03(0.70) | 6.07(0.73) | 0.02 | −0.04 |
| ≥7.5‐9.9% | SDiffb | SDiffb | ≥10‐19.9% | SDiffa | SDiffa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Statin nonusers | Statin users MPR<70% | Statin users MPR≥70% | Statin nonusers | Statin users MPR<70% | Statin users MPR≥70% | ||||
| Age, mean (SD) years | 61.74(6.85) | 61.34(7.11) | 61.02(7.14) | 0.06 | 0.1 | 64.85(5.82) | 64.32(6.24) | 63.93(6.41) | 0.09 | 0.15 |
| Sex (% women) | 16.23 | 16.49 | 17.19 | −0.01 | −0.03 | 9.46 | 9.63 | 9.99 | −0.01 | −0.02 |
| High‐risk alcohol intake (%) | 8.13 | 6.83 | 6.66 | 0.05 | 0.06 | 8.66 | 9.81 | 6.52 | −0.04 | 0.08 |
| Smokers (%) | 60.42 | 64.44 | 66.88 | −0.08 | −0.13 | 72.17 | 73.9 | 74.23 | −0.04 | −0.05 |
| Diabetes (%) | 21.53 | 19.82 | 19.32 | 0.04 | 0.06 | 33.34 | 31.71 | 31.09 | 0.03 | 0.05 |
| Hypertension (%) | 40.75 | 37.91 | 36.12 | 0.06 | 0.1 | 49.12 | 45.93 | 43.68 | 0.06 | 0.11 |
| Dyslipidemia (%) | 19.28 | 17.93 | 15.54 | 0.03 | 0.1 | 25.94 | 23.79 | 22.09 | 0.05 | 0.09 |
| Obesity (%) | 45.34 | 44.58 | 44.29 | 0.02 | 0.02 | 47.45 | 47.52 | 46.41 | 0 | 0.02 |
| Chronic kidney disease (%) | 0.89 | 0.85 | 0.76 | 0 | 0.02 | 1.23 | 1.18 | 1.15 | 0 | 0.01 |
| Blood pressure | ||||||||||
| Systolic, mean (SD), mm Hg | 140.79(15.81) | 141.24(16.38) | 141.41(16.56) | −0.03 | −0.04 | 144.60(15.72) | 144.81(16.25) | 144.93(16.23) | −0.01 | −0.02 |
| Diastolic, mean (SD), mm Hg | 82.24(10.19) | 82.42(10.14) | 82.41(9.79) | −0.02 | −0.02 | 82.52(10.00) | 82.92(10.13) | 83.10(10.13) | −0.04 | −0.06 |
| Total cholesterol, mean (SD), mmol/L | 5.77(0.72) | 5.86(0.81) | 5.88(0.83) | −0.11 | −0.14 | 5.88(0.77) | 5.97(0.77) | 6.01(0.86) | −0.12 | −0.17 |
| Total cholesterol, mean (SD), mg/dl | 223.16(27.94) | 226.48(31.47) | 227.25(32.23) | −0.11 | −0.13 | 227.21(29.76) | 230.68(29.66) | 232.42(33.07) | −0.12 | −0.16 |
| LDL cholesterol, mean (SD), mmol/L | 3.56(0.61) | 3.66(0.70) | 3.66(0.69) | −0.14 | −0.15 | 3.61(0.63) | 3.70(0.69) | 3.71(0.69) | −0.13 | −0.15 |
| LDL cholesterol, mean (SD), mg/dl | 137.83(23.54) | 141.38(27.10) | 141.55(26.86) | −0.14 | −0.14 | 139.72(24.21) | 142.92(26.50) | 143.50(26.72) | −0.13 | −0.14 |
| HDL cholesterol, mean (SD), mmol/L | 1.22(0.29) | 1.22(0.28) | 1.22(0.26) | −0.01 | −0.02 | 1.15(0.26) | 1.16(0.26) | 1.16(0.25) | 0 | 0 |
| HDL cholesterol, mean (SD), mg/dl | 47.17(11.21) | 47.26(10.82) | 47.34(10.03) | −0.01 | −0.01 | 44.66(9.95) | 44.69(10.04) | 44.71(9.51) | −0.00 | −0.00 |
| Serum triglycerides,mean (SD), mmol/L | 1.61(0.95) | 1.90(1.34) | 1.91(1.48) | −0.26 | −0.25 | 1.71(1.05) | 2.04(1.58) | 2.00(1.61) | −0.25 | −0.22 |
| Serum triglycerides,mean (SD), mg/dl | 142.18(84.47) | 168.52(118.53) | 169.61(131.15) | −0.26 | −0.24 | 151.03(92.73) | 180.93(140.15) | 177.12(142.49) | −0.25 | −0.21 |
| Glucose, mmol/L | 6.19(1.85) | 6.19(2.32) | 6.22(2.60) | 0.00 | −0.02 | 6.682.08 | 6.662.42 | 6.72.89 | 0.01 | −0.01 |
| Glucose, mg/dl | 111.4(33.31) | 111.43(41.75) | 112.01(46.86) | 0.00 | −0.02 | 120.1637.42 | 119.9143.63 | 120.5352.09 | 0.01 | −0.01 |
| Body Mass Index, Kg/m2 | 29.52(5.21) | 29.41(5.10) | 29.42(5.00) | 0.02 | 0.02 | 29.75.12 | 29.694.9 | 29.614.79 | 0.00 | 0.02 |
| 10‐year CHD risk, mean (SD) | 8.59(0.70) | 8.59(0.71) | 8.61(0.71) | −0.01 | −0.03 | 12.98(2.35) | 13.00(2.44) | 12.89(2.56) | −0.01 | 0.03 |
SDiff: standardized differences respect to nonusers.
SDiff: standardized differences respect to nonusers.
Outcomes and statin effectiveness
For 2006–2014, overall unadjusted incidences per 1,000 person‐years at risk (PYAR) of atherosclerotic cardiovascular disease (ASCVD), CHD, ischemic (IS), and all‐cause mortality were 2.98 (95% confidence interval (CI) 2.93–3.03), 2.60 (95% CI 2.55–2.64), 1.75 (95% CI 1.71–1.79), and 3.44 (95% CI 3.39–3.49), respectively. Unadjusted incidences and adjusted hazard ratios (HRs) for all outcomes are shown by CHD risk categories in Table 3. Statins showed a protective effect in individuals with MPR 70% for ASCVD across all risk groups, and this effect was significant in the risk groups defined from 7.5% through 9.9% and 10–19.9%; however, individuals with MPR <70% in any CHD risk category showed a lower and nonsignificant effect size reduction in ASCVD.
Table 3.
Adjusted hazard ratios (HR) of incident cardiovascular events and mortality and the 5‐year number needed to treat to prevent one event by statins use, by patients' coronary risk
| Statin nonusers | Statin users MPR <70% | Statin users MPR ≥70% | Statin users MPR <70% | Statin users MPR ≥70% | |||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Incidence (95% CI) | Events | Incidence (95% CI) | Events | Incidence (95% CI) | Adjusted HR(95% CI) | Adjusted HR(95% CI) | ||
| Outcomes of interest | 5‐year NNT | ||||||||
| <5% | |||||||||
| Composite ASCVD | 7113 | 2.04(1.76–2.31) | 153 | 3.74(3.08–4.39) | 150 | 3.58(2.94–4.21) | 0.91(0.74–1.13) | 0.74(0.52–1.04) | 470 |
| Coronary heart disease | 6136 | 1.76(1.51–2.01) | 138 | 3.37(2.73–4.01) | 149 | 3.55(2.97–4.14) | 0.90(0.73–1.12) | 0.80(0.57–1.13) | 400 |
| Ischemic stroke | 4228 | 1.21(1.05–1.37) | 88 | 2.14(1.68–2.61) | 98 | 2.32(1.81–2.82) | 0.84(0.65–1.09) | 0.75(0.51–1.11) | 838 |
| All‐cause mortality | 8604 | 2.44(2.28–2.60) | 111 | 2.67(2.10–3.24) | 125 | 2.94(2.39–3.49) | 0.85(0.66–1.08) | 0.83(0.55–1.27) | 668 |
| ≥5–7.4% | |||||||||
| Composite ASCVD | 2360 | 6.85(6.52–7.19) | 86 | 6.96(5.27–8.65) | 106 | 6.49(4.97–8.01) | 0.97(0.74–1.27) | 0.84(0.64–1.09) | 204 |
| Coronary heart disease | 2150 | 6.23(5.86–6.60) | 86 | 7.00(5.14–8.85) | 99 | 6.04(4.76–7.33) | 0.95(0.72–1.26) | 0.77(0.61–0.97) | 155 |
| Ischemic stroke | 1346 | 3.87(3.63–4.11) | 47 | 3.79(2.53–5.04) | 64 | 3.91(2.74–5.07) | 0.97(0.67–1.40) | 0.87(0.61–1.25) | 480 |
| All‐cause mortality | 2451 | 6.96(6.53–7.39) | 61 | 4.83(3.42–6.25) | 85 | 5.11(3.90–6.32) | 0.90(0.66–1.24) | 0.88(0.67–1.14) | 325 |
| ≥7.5–9.9% | |||||||||
| Composite ASCVD | 1355 | 9.99(9.30–10.68) | 63 | 9.86(6.90–12.82) | 81 | 8.43(6.44–10.41) | 0.89(0.63–1.27) | 0.70(0.54–0.92) | 75 |
| Coronary heart disease | 1148 | 8.42(7.60–9.24) | 63 | 9.78(6.85–12.71) | 78 | 8.11(6.19–10.02) | 0.93(0.66–1.30) | 0.71(0.54–0.94) | 90 |
| Ischemic stroke | 803 | 5.84(5.38–6.29) | 34 | 5.18(3.18–7.19) | 54 | 5.54(3.88–7.20) | 0.89(0.58–1.38) | 0.86(0.62–1.19) | 273 |
| All‐cause mortality | 1429 | 10.20(9.42–10.97) | 45 | 6.85(4.46–9.24) | 65 | 6.57(4.89–8.25) | 0.91(0.63–1.32) | 0.85(0.63–1.13) | 177 |
| ≥10–19.9% | |||||||||
| Composite ASCVD | 1391 | 13.34(12.57–14.1) | 105 | 14.15(11.30–17.00) | 151 | 12.04(10.08–14.01) | 0.96(0.77–1.20) | 0.74(0.61–0.90) | 62 |
| Coronary heart disease | 1241 | 11.84(11.10–12.58) | 86 | 11.61(8.96–14.26) | 142 | 11.27(9.36–13.18) | 0.83(0.64–1.07) | 0.74(0.60–0.92) | 71 |
| Ischemic stroke | 811 | 7.64(7.06–8.21) | 60 | 7.88(5.84–9.92) | 93 | 7.30(5.79–8.80) | 0.97(0.74–1.29) | 0.78(0.61–1.00) | 127 |
| All‐cause mortality | 1587 | 14.57(13.78–15.35) | 105 | 13.53(10.74–16.31) | 125 | 9.61(7.78–11.44) | 1.15(0.92–1.43) | 0.73(0.59–0.91) | 69 |
Incidence 1,000p/year. 5‐year NNT: calculated for statin users with MPR ≥70%.
Statins protected individuals with MPR 70% from CHD in the two risk groups defined from 5–9.9% and in the 10–19.9% risk group; however, individuals with MPR <70% showed no clinically relevant reduction in CHD in any risk category.
Furthermore, no significant differences in IS were found in any risk category, regardless of MPR. All‐cause mortality reduction was statistically significant only in individuals with MPR 70% and 10‐19.9% risk (0.73 (0.59–0.91)). Variables not balanced between statins users and nonusers were further included in the models but the results did not change (data not shown). Standard adjusted models showed similar results (Table S6).
Overall, 5‐year number needed to treat (NNT) was lower at higher levels of risk, ranging from 470 in the group with lowest CHD risk to 62 in the highest risk group.
Adverse events
There was no significant increase in cancer and hemorrhagic stroke attributable to statins, regardless of CHD risk category. However, diabetes increased in all CHD risk categories, although this was a nonsignificant trend in the 7.5–9.9% risk group (Table 4). Standard adjusted models showed similar results (Table S7).
Table 4.
Adjusted hazard ratios (HR) of adverse effects of statins use, by coronary risk of participants with adverse event
| Statin nonusers | Statin users MPR < 70% | Statin users MPR ≥ 70% | Statin users MPR < 70% | Statin users MPR ≥ 70% | ||||
|---|---|---|---|---|---|---|---|---|
| Events | Incidence (95% CI) | Events | Incidence (95% CI) | Events | Incidence (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95%CI) | |
| <5% | ||||||||
| Cancer | 21 077 | 7.04(6.54–7.54) | 312 | 8.97(7.90–10.04) | 414 | 11.68(10.51–12.85) | 0.91(0.80–1.03) | 1.06(0.87–1.30) |
| Type 2 diabetes | 20 922 | 7.18(6.45–7.92) | 394 | 12.23(10.96–13.50) | 515 | 16.29(14.77–17.80) | 0.99(0.86–1.14) | 1.15(0.91–1.46) |
| Hemorrhagic stroke | 1451 | 0.48(0.43–0.53) | 30 | 0.84(0.52–1.16) | 25 | 0.68(0.40–0.95) | 1.10(0.73–1.65) | 0.77(0.46–1.29) |
| Acute liver disease | 263 | 0.57(0.48–0.65) | 9 | — | 2 | — | — | — |
| Myopathy | 44 | 0.09(0.07–0.12) | 3 | — | 3 | — | — | — |
| ≥5–7.4% | ||||||||
| Cancer | 4306 | 14.68(14.12–15.25) | 124 | 11.80(9.18–14.42) | 188 | 13.53(11.41–15.65) | 0.94(0.75–1.18) | 1.02(0.85–1.22) |
| Type 2 diabetes | 4549 | 18.35(17.72–18.99) | 170 | 21.29(17.45–25.12) | 258 | 26.87(23.19–30.54) | 1.12(0.91–1.37) | 1.32(1.12–1.56) |
| Hemorrhagic stroke | 396 | 1.30(1.16–1.44) | 12 | 1.11(0.37–1.85) | 14 | 1.00(0.38–1.62) | 0.92(0.45–1.85) | 0.74(0.39–1.41) |
| Acute liver disease | 48 | 1.02(0.64–1.40) | 2 | — | 2 | — | — | — |
| Myopathy | 8 | — | 1 | — | 1 | — | — | — |
| ≥7.5–9.9% | ||||||||
| Cancer | 2247 | 19.50(18.50–20.49) | 90 | 16.56(12.27–20.84) | 128 | 15.55(12.70–18.39) | 1.01(0.79–1.30) | 0.92(0.74–1.13) |
| Type 2 diabetes | 1939 | 22.15(21.05–23.26) | 97 | 27.28(20.88–33.68) | 142 | 29.38(23.47–35.28) | 1.19(0.92–1.54) | 1.20(0.96–1.49) |
| Hemorrhagic stroke | 221 | 1.83(1.57–2.09) | 11 | 1.93(0.64–3.22) | 18 | 2.10(0.95–3.26) | 1.16(0.57–2.34) | 1.16(0.62–2.15) |
| Acute liver disease | 26 | 1.36(0.72–2.01) | 3 | — | 1 | — | — | — |
| Myopathy | 4 | — | 3 | — | 1 | — | — | — |
| ≥10–19.9% | ||||||||
| Cancer | 2151 | 24.33(22.99–25.67) | 136 | 21.46(17.58–25.33) | 228 | 21.38(18.43–24.34) | 1.06(0.87–1.30) | 1.00(0.85–1.18) |
| Type 2 diabetes | 1490 | 25.80(24.24–27.35) | 101 | 29.38(23.03–35.73) | 172 | 34.77(29.20–40.35) | 1.15(0.90–1.47) | 1.26(1.04–1.52) |
| Hemorrhagic stroke | 245 | 2.62(2.26–2.98) | 16 | 2.48(1.24–3.73) | 30 | 2.70(1.70–3.69) | 0.90(0.51–1.57) | 0.83(0.53–1.32) |
| Acute liver disease | 19 | 1.30(0.62–1.97) | 7 | — | 2 | — | — | — |
| Myopathy | 4 | — | 3 | — | 0 | — | — | — |
Incidence 1,000p/year.
Tables S8 and S9 show statin effectiveness and adverse effects in the complete dataset. The results of the proportional hazards assumption was met for all outcomes except for diabetes in the <5% category (Table S10).
DISCUSSION
This observational retrospective study aimed at estimating statin effectiveness according to CHD risk levels in a general population without previous cardiovascular disease. We found that statin treatment was effective in preventing ASCVD across the range of coronary risk in people with high adherence to therapy (MPR >70%). Specifically, statin therapy decreased ASCVD risk by 30% and 26% in individuals with cardiovascular risk of 7.5–9.9% and 10–19.9%, respectively. Treatment with statins also decreased all‐cause mortality by 27% in those at highest risk (10–19.9% category). We found no clinically relevant effect size of statin treatment in people with low adherence to therapy (MPR <70%), irrespective of their CHD risk.
Our finding that statins effectively reduced ASCVD in individuals with 10‐year CHD risk 10–19.9% who adhered to treatment is consistent with clinical trials and systematic reviews aimed at evaluating statin efficacy in primary prevention6, 7 and in line with recent guidelines.1, 2, 3, 4 In addition, our estimate of 62 as the 5‐year NNT to prevent one ASCVD event in the 10–19.9% risk category was similar to the results of the Cholesterol Trialists Collaborators' meta‐analysis in primary prevention, which included individuals with 10‐year coronary risk of about 15%, similar to the individuals in our 10–19.9% category.6 Hence, statin therapy may be useful in this CHD risk category.
We found that statin treatment effectively reduced relative risk in the 7.5–9.9% category, similar to that of the cited meta‐analysis with a pooled population comparable to ours in this 10‐year risk category.5 Although our 5‐year NNT estimate in this risk category was about 20% higher compared to the 10–19.9% category (75 vs. 62), most individuals with an estimated CHD risk 7.5–9.9% could benefit from statin treatment, in line with US guidelines.1, 2 Additional research is needed to define strategies that improve the predictive ability of risk functions by considering new risk factors13 and/or by individualized consideration of LDL cholesterol levels or response to treatment.14, 15
Patient management strategies must take into account the net benefit of absolute risk reduction,10, 14 long‐term treatment safety, costs, and patient preference9, 16 in the 10–19.9% and 7.5–9.9% categories.
The relative benefit for protection against ASCVD in the 5–7.4% risk category in individuals with MPR 70% was mainly due to the protective effect for CHD. This finding is in accordance with the results of a recent pragmatic clinical trial in an intermediate‐risk population, defined as a 10‐year cardiovascular risk of about 10% but 10‐year CHD risk of about 5%.7 However, the net benefit of statin treatment in the <5% and 5–7.4% categories could be limited because of the large 5‐year NNT to prevent one event (470 and 204, respectively).
In our study, the magnitude of the statin effectiveness in preventing IS was similar to that of clinical trials and meta‐analyses.6, 17 It is likely that the few IS events contributed to the lack of statistical significance in our results. However, the lack of significant association between statin treatment and IS was in line with previous observational data.18
There is also great debate about the effectiveness of statins on all‐cause mortality,6, 7, 19, 20 despite agreement that the relative risk reduction is moderate (∼10%). Our findings were consistent with this effect size in all risk categories except for the association between statins and all‐cause mortality in individuals with MPR 70% and 10–19.9% risk. The effect was greater than expected. We cannot dismiss the possibility of residual healthy‐user effect, although this was not observed in other risk categories.
About 46% of new users had a 6‐month MPR <70%. Importantly, patients with poor adherence are more likely to have a higher incidence of cardiovascular and cerebrovascular events and all‐cause mortality than adherent patients.21 Hence, a first‐line strategy to improve statin effectiveness in primary care should be the implementation of interventions to improve adherence.
Adverse effects
Statin treatment in users with MPR 70% increased the risk of diabetes from 15% to 32% depending on risk category, consistent with results from clinical trials.22 This excess risk should be considered in recommendations for lifetime statin therapy. We observed no increased risk of cancer or hemorrhagic stroke among new users of statins, which is also consistent with the literature.23, 24
Strengths and limitations
A major strength of this study is that it was based on high‐quality, internally validated electronic medical records that provided a large sample size, ensured high external validity,12 and reflected real‐life clinical conditions by including individuals often excluded from clinical trials (e.g., women, diabetics, people with inflammatory immune disorders).
Ten‐year coronary risk was stratified by an equation validated in Spain.25 The concordance between the estimated and observed 10‐year coronary risk in our study reinforces the quality of this risk equation.
We acknowledge several limitations. First, residual confounding is a possibility, especially indication bias26; we excluded frail individuals and used a new users design to minimize confounding factors and then adjusted for PS in each coronary risk stratum. The exclusion of frail individuals may also lead to an unrepresentative sample; however, the population of greatest interest consists of individuals more likely to receive statins in primary prevention and frail individuals are less likely to initiate statin treatment. The inclusion of prevalent users may also lead to a sample whose covariates at baseline, especially lipid profile, could be affected by previous statin use.
Second, missing data can influence results. To avoid this type of selection bias, where the population with missing data somehow differs from those with complete data, we imputed the missing values for continuous variables instead of excluding those records. Overall, the population with complete data was more likely to be older, female, hypertensive, and diabetic (Supplementary file, Table S4). The percentage of missing data ranged from 28–79%. The appropriateness of performing multiple imputations depends not only on the percentage and mechanism of missing values but on the number of complete observations used in the imputation process. In our study, 77,894 complete cases were available to impute variables with missing values, increasing the likelihood of representing the general population.
Third, we could not analyze the effect of statins on cardiovascular death, as cause‐of‐death is not available in the SIDIAPQ database. Fourth, we cannot exclude some underreporting of outcomes, which could lead to nondifferential misclassification and reduce statistical power, biasing results towards the null hypothesis. There were too few cases to accurately estimate the effect of statins use on acute liver disease and myopathy incidence or on relative and absolute risk reduction.
In addition, follow‐up was shorter than the 10‐year CHD risk; that may result in a misclassification of individuals in each CHD risk category in the long term. We also estimated 10‐year CHD incidence based on the observed data using a Weibull model, which showed that the 10‐year mean incidence of CHD in each risk category matched the Framingham score estimates (Table S11). Low data quality could also generate misclassification. In this study the presence of cardiovascular risk factors and outcomes were previously validated in SIDIAP.27 Moreover, the validity of statins exposure in the medical records was confirmed by the official invoicing records from community pharmacies.
Lastly, we could not analyze statin effectiveness in subgroups based on statin dose or sex because of the few events in the high‐dose category and among women in the highest coronary risk categories.
In conclusion, statin treatment in adherent patients was associated with a reduction in ASCVD risk ranging from 16–30%. The 5‐year NNT in the 5–7.4% and 7.5–9.9% CHD risk categories was higher (204 and 75, respectively) than in the 10–19.9% CHD risk category (5‐year NNT: 62). Statins were less effective in preventing IS, compared to CHD. All‐cause mortality was significant only in the 10–19.9% CHD risk category (HR: 0.73 (0.59–0.91)).
Our results indicate that interventions in primary care should focus on improving adherence to statin therapy. Statin treatment should remain a priority in managing patients at high 10‐year CHD risk (10–19.9%). In the same way, most patients in the 7.5–9.9% category could benefit from statin treatment, although the increased risk of diabetes, costs, and patient preference must be taken into account. In the 5–7.4% range, the higher 5‐year NNT (204) raises the question of whether it is reasonable to recommend treatment in these patients.
METHODS
Study design
This was a historical population‐based cohort study.
Record linkage system
The Information System for the Development of Research in Primary Care (SIDIAP) is a clinical database of anonymized longitudinal patient records for nearly six million people (80% of the Catalan population and 10.2% of the total population of Spain) registered in 274 primary care practices having a total of 3,414 general practitioners (GPs). A subset of records from GPs who surpass a predefined data quality standard constitutes the SIDIAPQ, which provides research‐quality anonymized clinical data covering 14 million person‐years (2005–2014). SIDIAPQ is highly representative of both urban and rural areas of Catalonia.28
The quality of SIDIAPQ data has been previously documented, and the database has been used to study the epidemiology of a number of health outcomes.13, 27, 29 Ethics approval was obtained from our local Ethics Committee.
Study population
All individuals aged 35 to 74 years were included in SIDIAPQ.
Inclusion criteria
Only new users (defined as receiving simvastatin, pravastatin, lovastatin, fluvastatin, rosuvastatin, or atorvastatin for the first time) were selected. For this group, the index date was defined as the first statin invoice; those same dates were then randomly assigned to nonusers to achieve a similar distribution. Individuals with at least one visit to their healthcare provider within 18 months before the index date were included.
Exclusion criteria
Frail individuals with cancer, dementia, paralysis, organ transplant, on dialysis, or institutionalized at baseline were excluded, as were patients with previous history of peripheral arterial disease, CHD, IS or hemorrhagic stroke, revascularization, heart failure or cardiac therapy (ATC code C01), or cholesterol‐lowering drugs other than statins taken between July 2006 and December 2007. Individuals with <2 invoices in pharmacological records for statins during the enrollment period were also excluded. Finally, we excluded individuals with estimated CHD risk 20% (n = 1,561) because statins are clearly cost‐effective in this risk category in terms of absolute risk reduction and safety.1, 2, 3, 4
Statin exposure
Statin exposure was calculated according to the medical possession ratio (MPR), defined as the number of days of statin supplied according to pharmaceutical records during 6 consecutive months, divided by 183 days. Statin users were categorized as low (MPR <70%) or high (MPR 70%) adherence to therapy.
Outcomes
Cardiovascular diseases during follow‐up were identified from SIDIAPQ codes in both primary care (ICD‐10) and hospital discharge records (ICD‐9). Outcomes were CHD (a composite of acute myocardial infarction (fatal and nonfatal AMI) and angina), fatal and nonfatal ischemic stroke (IS), and all‐cause mortality. The primary outcome of the study was ASCVD a composite of AMI and IS. Entry of cardiovascular codes in SIDIAPQ has been previously validated.27
Adverse effects
Liver toxicity and myopathy were attributed to statins if these effects occurred within 12 months of initiating treatment. New‐onset diabetes, cancer, and hemorrhagic stroke were attributed to statin exposure if the diagnosis occurred at least 1 year after the prescription date.30
Study entry and follow‐up
Patients were enrolled from July 2006 to December 2007 and censored at the date of transfer from SIDIAPQ or end of study, December 31, 2014. Baseline period was defined as 1 year previous to the index date.
Baseline covariates
The following covariates at baseline were considered: 10‐year coronary risk was calculated using the 10‐year CHD risk Framingham function adapted and validated in the Spanish population.25 Local guidelines31 recommend the use of this function, which allows the estimation of CHD risk in people without previous CVD up to the age of 74 years and includes fatal and nonfatal coronary events. Individuals were categorized to assess benefit in the low‐intermediate and high‐intermediate coronary risk populations, as follows: <5%, 5–7.4%, 7.5–9.9%. and 10–19.9%.
Statistical analysis
Results are expressed as percentages for categorical variables and as mean (SD), or median (quartiles) otherwise. Under the missing‐at‐random assumption, we used multiple imputations by chained equations32 to replace missing baseline values for total cholesterol, high‐density lipoprotein cholesterol, triglycerides, glucose, systolic and diastolic blood pressure, BMI (weight and height), and deprivation index score. The 10‐year coronary risk was calculated and individuals were then classified into the study's four coronary risk categories. The process of multiple imputation and the variables considered in the models are detailed in the Supplementary file. Because of nonrandom treatment allocation, a multinomial model based on potential confounding covariates (Table 5) was used to calculate a PS for statin therapy (PS model development and assessment are shown in the Supplementary file). Baseline characteristics of the groups before and after adjusting for PS were compared using standardized differences. Variables with standardized differences <0.10 were considered well‐balanced.
Table 5.
Baseline covariates
| Sociodemographic characteristics |
|
| Cardiovascular risk factors |
|
| Measurements |
|
| Record of other drug use |
|
| Other comorbidities (ICD‐10 codes: yes/no) |
|
ICD‐10, International Classification of Disease, 10th revision; BMI, body mass index.
The study used the deprivation index derived from the multicenter MEDEA study in Spain. The index is described in detail in the Supplementary file.
Ten PS and 10 hazard ratio (HR) values were calculated for each coronary risk group in each imputed dataset. A pooled HR was then calculated according to Rubin's rules32 with PS as covariate. Variables not balanced between statins users and nonusers were further included in the models. In a sensitivity analysis, we compared complete‐case results with those of multiple imputation and standard adjusted models were also performed (Supplementary file).
Proportionality of hazards assumption was tested by calculating the median of the chi‐square tests of the models fitted for the 10 imputed datasets (Supplementary file). Five‐year NNT for one additional patient to survive to a specific timepoint was also calculated. Statistical analysis used R software.33
Ethics approval
Ethics approval was obtained from our local Ethics Committee (IDIAP Jordi Gol) and all procedures were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
CONFLICT OF INTEREST
No author has reported any potential conflicts of interest involving the present work. Drs. Ramos and Garcia‐Gil reported that they collaborate in projects of IDIAP. Jordi Gol is funded by AstraZeneca and AMGEN. These projects are unrelated to the present work.
FUNDING
This work was supported by grants from the Spanish government's 2010 Health Ministry call for clinical research proposals (EC10‐084), the 2012 Ministry of Science and Innovation call through the Carlos III Health Institute (Nets RD12/0005/0002, RD12/0042), and the 2013 call from the Carlos III Health Institute for clinical research proposals related to the Health Strategic Action Plan, 2013‐2016, under the Program on Research Related to Society's Challenges, within the framework of Spain's 2013‐2016 Plan for Scientific and Technical Research and Innovation (PI13/01511, cofunded by the European Fund for Regional Development (EFRD) of the European Union). M.G. is funded by a FEDER contract from Carlos III Health institute (FIS CP12/03287).
AUTHOR CONTRIBUTIONS
M.G.G., R.R., J.M., and I.P. wrote the article; M.G.G., R.R., J.M., J.B., and M.C. designed the research; M.G.G., R.R., J.M., I.P., L.A., A.P., R.M., R.E., J.B., M.C., and M.G. performed the research; J.B. and M.C. analyzed the data.
Supporting information
Supplementary File
ACKNOWLEDGMENTS
For data on Hospital Discharges, we thank the Registre del conjunt mínim de bases de dades (CMBD), Divisió de Registres de Demanda i d'Activitat, Àrea de Serveis i Qualitat, Servei Català de la Salut. This paper was not prepared in collaboration with CMBD personnel and does not necessarily reflect the agency's opinion or point of view. The authors take sole responsibility for the integrity of the data and the accuracy of the analysis. We thank Eduardo Hermosilla for data management support. We also appreciate the revision of the English text by Elaine Lilly, Ph.D.
This article was published online on 02 February 2018. An error was subsequently identified in the authors' affiliations section and a new affiliation was added for the authors Jaume Marrugat, Roberto Elosua, and Maria Grau. This notice is included in the online and print versions to indicate that both have been corrected on 14 February 2018.
References
- 1. Stone, N.J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2889–934 (2014). [DOI] [PubMed] [Google Scholar]
- 2. US Preventive Services Task Force . Statin use for primary prevention of cardiovascular disease in adults. JAMA 316, 1997–2007 (2016). [DOI] [PubMed] [Google Scholar]
- 3. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. <https://www.nice.org.uk/guidance/cg67?unlid=530308905201656193829>.
- 4. Catapano, A.L. et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2999–3058 (2016). 27567407 [Google Scholar]
- 5. Cholesterol Treatment Trialists' (CTT) Collaboration , Baigent, C. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cholesterol Treatment Trialists' (CTT) Collaborators , Mihaylova, B . et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet 380, 581–590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yusuf, S . et al; HOPE‐3 Investigators . Cholesterol lowering in intermediate‐risk persons without cardiovascular disease. N. Engl. J. Med. 374, 2021–2031 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Ray, K.K . et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur. Heart J. 35, 960–968 (2014). [DOI] [PubMed] [Google Scholar]
- 9. Wise, J . Open letter raises concerns about NICE guidance on statins. BMJ 348, g3937 (2014). [DOI] [PubMed] [Google Scholar]
- 10. Tresidder A. NICE should publish numbers needed to treat and harm for statins. BMJ 348, g3458 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Mills, E.J. et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta‐analysis involving more than 65,000 patients. J. Am. Coll. Cardiol. 52, 1769–1781 (2008). [DOI] [PubMed] [Google Scholar]
- 12. Tannen, R.L. , Weiner, M.G. & Xie, D. Use of primary care electronic medical record database in drug efficacy research on cardiovascular outcomes: comparison of database and randomised controlled trial findings. BMJ 38, b81 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramos, R. M , et al. Statins for prevention of cardiovascular events in a low‐risk population with low ankle brachial index. J. Am. Coll. Cardiol. 67, 630–640 (2016). [DOI] [PubMed] [Google Scholar]
- 14. Soran, H. , Schofield, J.D. & Durrington, P.N. The importance of considering LDL cholesterol response as well as cardiovascular risk in deciding who can benefit from statin therapy. Curr. Opin. Lipidol. 25, 239–246 (2014). [DOI] [PubMed] [Google Scholar]
- 15. Thanassoulis, G . et al. Individualized statin benefit for determining statin eligibility in the primary prevention of cardiovascular disease. Circulation 133, 1574–1581 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Greving, J.P. , Visseren, F.L.J. , de Wit, G.A. & Algra, A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost‐effectiveness analysis. BMJ 342, d1672 (2011). [DOI] [PubMed] [Google Scholar]
- 17. Briel, M. , Studer, M. , Glass, T.R. & Bucher, H.C. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta‐analysis of randomized controlled trials. Am. J. Med. 117, 596–606 (2004). [DOI] [PubMed] [Google Scholar]
- 18. Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration. Lancet 346, 1647–1653 (1995). [PubMed] [Google Scholar]
- 19. Ray, K.K. et al. Statins and all‐cause mortality in high‐risk primary prevention: a meta‐analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 170, 1024–1031 (2010). [DOI] [PubMed] [Google Scholar]
- 20. Naci, H . et al. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all‐cause mortality: a network meta‐analysis of placebo‐controlled and active‐comparator trials. Eur. J. Prev. Cardiol. 20, 641–657 (2013). [DOI] [PubMed] [Google Scholar]
- 21. Slejko, J.F. et al. Adherence to statins in primary prevention: yearly adherence changes and outcomes. J. Manag. Care Pharm. 20, 51–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sattar, N . et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet 375, 735–742 (2010). [DOI] [PubMed] [Google Scholar]
- 23. Cholesterol Treatment Trialists' (CTT) Collaboration , Emberson, J.R. et al. Lack of effect of lowering LDL cholesterol on cancer: meta‐analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 7, e29849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKinney, J.S. & Kostis, W.J. Statin therapy and the risk of intracerebral hemorrhage: a meta‐analysis of 31 randomized controlled trials. Stroke 43, 2149–2156 (2012). [DOI] [PubMed] [Google Scholar]
- 25. Marrugat, J . et al. Relative validity of the 10‐year cardiovascular risk estimate in a population cohort of the REGICOR study. Rev. Esp. Cardiol. 64, 385–394 (2011). [DOI] [PubMed] [Google Scholar]
- 26. Mann, D.M. , Woodward, M. , Muntner, P. , Falzon, L. & Kronish, I. Predictors of nonadherence to statins: a systematic review and meta‐analysis. Ann. Pharmacother. 44, 1410–1421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos, R. et al. Validity for use in research on vascular diseases of the SIDIAP (Information System for the Development of Research in Primary Care): the EMMA study. Rev. Española Cardiol. 65, 29–37 (2012). [DOI] [PubMed] [Google Scholar]
- 28. García‐Gil, M.M. et al. Construction and validation of a scoring system for the selection of high‐quality data in a Spanish population primary care database (SIDIAP). Qual. Prim. Care 20, 135–145 (2012). [DOI] [PubMed] [Google Scholar]
- 29. García‐Gil, M. et al. Patterns of statin use and cholesterol goal attainment in a high‐risk cardiovascular population: a retrospective study of primary care electronic medical records. J. Clin. Lipidol. 10, 134–142 (2015). [DOI] [PubMed] [Google Scholar]
- 30. Smeeth, L. , Douglas, I. , Hall, A.J. , Hubbard, R. & Evans, S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br. J. Clin. Pharmacol. 67, 99–109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baena Díez, J.M. et al. Colesterol i risc coronari Barcelona: Institut Català de la Salut, 2009. Guies de pràctica clínica i material docent, núm. <http://www.gencat.cat/ics/professionals/guies/colesterol/colesterol.htm>.
- 32. White, I.R. , Royston, P. & Wood, A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 30, 377–399 (2011). [DOI] [PubMed] [Google Scholar]
- 33. Team RDC . R: A language and environment for statistical computing. R Foundation for Statistical Computing (Internet) Vienna, Austria; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File


