Abstract
Purpose
To investigate risk factors for the development and progression of diabetic retinopathy (DR) and long‐term visual outcomes in Dutch patients with type 1 diabetes mellitus (T1DM).
Methods
Cumulative incidences were calculated for DR, vision‐threatening DR (VTDR), defined as (pre)proliferative DR and diabetic macular oedema, and best‐corrected visual acuity (BCVA) <0.5 and <0.3 at the most recent eye examination. The following factors were assessed: duration of diabetes, age of onset of T1DM, gender, mean HbA1c, HbA1c variability (defined as coefficient of variation of five separate HbA1c measurements), mean arterial blood pressure, body mass index, albuminuria and lipid profile. We used multivariable Cox regression models to identify factors associated with DR development and progression to VTDR.
Results
We found 25‐year cumulative incidences of 63% for DR, 21% for VTDR, 2% for BCVA <0.5, and 1% for BCVA <0.3. Mean HbA1c (HR 1.023, p < 0.001), HbA1c variability (HR 1.054, p < 0.001), age of onset of T1DM (HR 1.024, p < 0.001), HDL cholesterol (HR 0.502, p = 0.002) and total cholesterol (HR 1.210, p = 0.029) showed an independent association with faster development of any form of DR. The mean HbA1c (HR 1.023, p < 0.001) and the presence of albuminuria (HR 2.940, p = 0.028) were associated with faster progression to VTDR.
Conclusion
These data show relatively low cumulative incidences of DR, VTDR and visual impairment. Higher mean HbA1c, HbA1c variability, age of onset of T1DM and total cholesterol were independently associated with the risk of DR development, and a protective association was found for HDL cholesterol in subjects with T1DM. Mean HbA1c and presence of albuminuria were associated with progression of DR.
Keywords: cumulative incidence, diabetic retinopathy, risk factors, type 1 diabetes mellitus, vision‐threatening diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is a potentially sight threatening microvascular complication of diabetes mellitus (DM), affecting a third of all DM patients worldwide (Ding & Wong 2012; Yau et al. 2012). Diabetic retinopathy (DR) is characterized by retinal microvascular damage, based on ischaemic changes and increased capillary permeability. Visual function can be affected by diabetic macular oedema (DME), macular ischaemia or as sequelae of proliferative diabetic retinopathy (PDR), notably vitreous haemorrhage and tractional retinal detachment. The substantial impact of DR on visual performance requires attention to prevention and early detection of this sight threatening condition.
Diabetic retinopathy (DR) is a complex multifactorial disease and many risk factors are involved. The most obvious and important predictive factor for the development and progression of DR is hyperglycaemia. The Diabetes Control and Complications Trial clearly showed that very intensive glycaemic control can reduce the incidence of DR by 76% and the progression of DR by 54% (DCCT, 1993). Other major systemic risk factors include hypertension, dyslipidaemia and high body mass index, although there is substantial variation in the consistency and strength of the association with these risk factors (Miljanovic et al. 2004; Ding & Wong 2012; Yau et al. 2012). Risk of development and progression of DR is furthermore influenced by a number of non‐modifiable risk factors, such as duration of diabetes, pregnancy, puberty and population‐based diversity (Raymond et al. 2009; Yau et al. 2012).
To the best of our knowledge, there are currently no cohort studies on development and progression of DR, and associate risk factors in Dutch patients with T1DM. The purpose of this study was to further explore the risk factors for the development and progression of DR in this patient population. In addition, we evaluated the long‐term visual outcomes in these patients.
Patients and Methods
Subjects
This study was conducted in a cohort of patients with T1DM who were recruited at the outpatient diabetes clinic of the Internal Medicine Department at the Radboud University Medical Center between 2006 and 2008 for a study on hypoglycaemia awareness (Sejling et al. 2016). Patients were eligible for the current analysis when the DR screen had take place at the Department of Ophthalmology of the Radboud University Medical Center. This study adhered to the tenets of the Declaration of Helsinki, and was approved by the local Institutional Review Board. Study participants provided written informed consent.
Eye determinants
Patients underwent complete ophthalmological examinations yearly or more often in those at high risk of visual decline. Examinations were standardized and included Snellen best‐corrected visual acuity (BCVA) and retinal evaluation with biomicroscopy. In case of any signs of DR or DME, diagnosis was confirmed with colour fundus photography (Topcon TRC 50 IX, Topcon Corporation, Tokyo, Japan) and optical coherence tomography (Spectralis™ HRA+OCT, Heidelberg Engineering, Heidelberg, Germany). Stage of DR and DME were determined according to the International Clinical Diabetic Retinopathy Severity Scale (Wilkinson et al. 2003). The eye with the most severe retinopathy was included in further analyses. The two most severe stages of DR/DME of the International Clinical Diabetic Retinopathy/Macular Edema Severity Scale were defined as vision‐threatening DR (VTDR) that is DR corresponding with the 4‐2‐1 rule or proliferative DR, retinal oedema or hard exudates approaching or involving the fovea. Non‐vision‐threatening DR (NVTDR) was defined as mild or moderate non‐proliferative DR or DME distant from the fovea.
Visual impairment was defined as a BCVA <0.3 in the best seeing eye according WHO International Statistical Classification of Diseases and Health Problems (WHO, 2015). We also used BCVA <0.5 as a cut‐off, as this is below the visual requirement for a European driving license (European Union). In addition to the presence of DR/DME and BCVA, all treatments which were administered during the study period were registered.
Data collection
We assessed the variables age at diagnosis of T1DM, gender and T1DM duration from medical charts. Serum measurements were obtained at first diagnosis of DR, or within 6 months around the date of the most recent eye examination in those without DR. The mean glycated haemoglobin (HbA1c) level was calculated as the average of 5 measurements at 3‐month intervals. The coefficient of variation (CV), a measure of HbA1c variability, was calculated from the mean and standard deviation of the HbA1c measurements (Luk et al. 2013). Other laboratory measurements were total cholesterol (mmol/l), high‐density lipoprotein (HDL) cholesterol (mmol/l) and albuminuria (mg/l). Albuminuria, the most important marker of diabetic nephropathy, was defined as a urinary albumin excretion of ≥30 mg/l in the absence of other renal pathology (American Diabetes Association, 2008). The mean arterial pressure was (MAP, mmHg) assessed by the equation: MAP ≃ diastolic blood pressure + ⅓ (systolic blood pressure – diastolic blood pressure); body mass index (BMI, kg/m2) was calculated as weight/height2. Medical files were assessed between April and July 2015.
Statistical analysis
For continuous variables, values were displayed as mean ± standard deviation (SD) for normal distributions, and as median with corresponding interquartile range (IQR) for skewed distributions. For categorical variables, values were presented as proportions in percentages. Binary logistic regression analysis was used to compare patient characteristics of the NVTDR group and the VTDR group with the no DR group. Time of follow‐up was defined as the time in years after first diagnosis of diabetes mellitus. Primary outcome variable was the presence of DR, secondary outcomes were VTDR, BCVA <0.5 and BCVA <0.3. Cumulative incidences of outcome variables were estimated using the Kaplan–Meier survival analysis. Values at the extreme end were pooled due to limited numbers. Incidence rate was calculated by dividing the number of new cases by the total number of person‐years.
We used a multiple imputation approach for randomly missing data with 20 iterations, incorporating both determinants and outcome variables. Multivariable Cox proportional hazards regression was used to identify predictors for DR and VTDR, with backward stepwise selection eliminating variables with p ≥ 0.1. Risks were displayed as hazard ratios (HR) with corresponding 95% confidence interval (CI). Survival curves displaying disease free fraction were plotted for low‐ and high‐risk profiles. Profiles were based on significant variables from the Cox analyses using cut‐off levels according to established clinical criteria. For variables for which standardized reference levels were not available, 25th and 75th percentiles were used to represent low and high risk. Using these criteria, low risk was defined as HbA1c = 53 mmol/mol, total cholesterol = 5.2 mmol/l and HDL = 1.6 mmol/l, HbA1c variability = 4.9 and age of onset of T1DM = 15 years. High risk was defined as HbA1c = 74 mmol/mol, total cholesterol = 6.2 mmol/l and HDL = 1.0 mmol/mol, HbA1c variability = 9.1 and age of onset of T1DM = 30 years (DCCT, 1993, National Institutes of Health, 2001; Feldman et al. 2014).
To obtain insight in the discriminative ability of our models, a risk score was calculated for each individual by multiplying the estimated beta coefficients from multivariate Cox regression analysis with the registered data points. Subsequently, risk scores were categorized and plotted in a histogram for no DR versus DR, and for NVTDR versus VTDR. All statistical analyses were conducted using SPSS version 20 (SPSS, Chicago, IL, USA).
Results
A total of 415 subjects were eligible for the current study. The follow‐up time varied from 7 to 65 years, with a median of 29 years. Total number of patients who developed DR was 284 (68%). Of these, 119 (42%) progressed to VTDR. Patient characteristics of the three groups are displayed in Table 1. As expected, duration of T1DM was longer in patients with NVTDR and VTDR than in patients without DR, and age of T1DM onset was lowest in the VTDR group. HbA1c determinants and blood pressure were less favourable in the NVTDR and VTDR groups, and HDL cholesterol was lowest in VTDR.
Table 1.
Patient characteristics
| Variables | No DR (n = 131) | NVTDR (n = 164) | VTDR (n = 120) |
|---|---|---|---|
| Age, years | 48 ± 37 | 52 ± 14 | 56 ± 13a |
| Male gender, n (%)c | 60 (46%) | 80 (49%) | 55 (46%) |
| Age of onset of T1DM, years | 23 ± 11 | 21 ± 12 | 18 ± 13b |
| Duration of T1DM, years | 22 ± 10 | 32 ± 12b | 38 ± 11b |
| Mean HbA1c, mmol/mold | 61 (56–67) | 65 (57–72)a | 78 (67–89)b |
| HbA1c variability, CVd | 5.9 (4.4–8.1) | 6.9 (5.0–9.1)a | 7.8 (5.8–11.0)b |
| Mean arterial pressure, mmHg | 91 ± 10 | 95 ± 10a | 96 ± 11a |
| Body mass index, kg/m2 d | 25 (22–27) | 26 (23–28) | 24 (22–27) |
| HDL cholesterol, mmol/l | 1.56 ± 0.37 | 1.46 ± 0.42 | 1.38 ± 0.35a |
| Total cholesterol, mmol/l | 4.9 ± 1.0 | 4.9 ± 0.8 | 5.1 ± 1.0 |
| Albuminuria, n (%)c | 10 (10%) | 7 (7%) | 6 (15%) |
Data are means ± SD.
DR = diabetic retinopathy; NVTDR = non‐vision‐threatening diabetic retinopathy; VTDR = vision‐threatening diabetic retinopathy; n = number; T1DM = type 1 diabetes mellitus; CV = coefficient of variation; HDL = high‐density lipoprotein.
p‐values addressed differences between no DR versus NVTDR/VTDR and were adjusted for age and gender.
p < 0.05.
p < 0.001.
Data are number of subjects with %.
Data are median with interquartile range.
The overall incidence rate of DR was 0.033 per person‐years and 0.014 per person‐years for VTDR. The 10‐year cumulative incidence of DR was 5%, 20‐year cumulative incidence 43%, 25‐year cumulative incidence 63%, and 30‐ and 40‐year cumulative incidences were 75% and 85%. For VTDR, the 20‐year cumulative incidence was 10%, 25‐year cumulative incidence 21%, and 30‐ and 40‐year cumulative incidences were, respectively, 34% and 51%. Fifty per cent of the total study population had developed DR after 22 years duration of DM and VTDR after 40 years (Fig. 1A). With respect to visual acuity, 2% developed BCVA <0.5 after 25 years of T1DM and 28% after 58 years; 1% developed BCVA <0.3 after 25 years of T1DM and 8% after 58 years (Fig. 1B), during which period the treatments were applied that are shown in Table S1.
Figure 1.
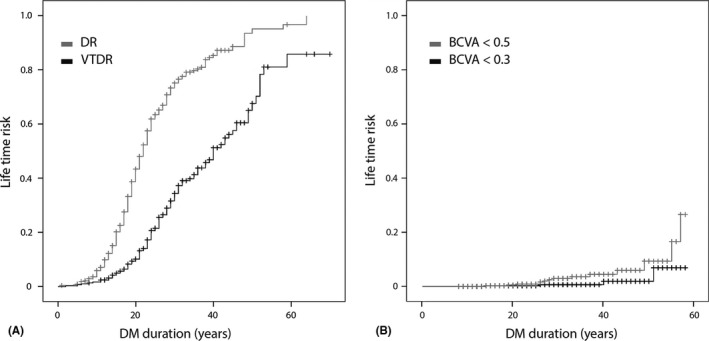
Lifetime risk as a function of DM duration for: (A) DR and VTDR; (B) visual impairment. DR = diabetic retinopathy; VTDR = vision‐threatening diabetic retinopathy; DM = diabetes mellitus; BCVA = best‐corrected visual acuity.
Laboratory assessments which coincided with eye examinations were available in 380 subjects (126 without DR, 158 NVTDR and 96 VTDR). In the multivariate Cox regression analysis based on these subjects, higher age of onset of T1DM, mean HbA1c, HbA1c variability, total cholesterol and low HDL cholesterol were associated with a faster development of DR (Table 2). The mean HbA1c and the presence of albuminuria were associated with a faster progression of DR to VTDR (Table 3). We compared the hazard of DR development for high‐ and low‐risk profiles with Cox regression survival curves (Fig. 2). Remarkably, high‐risk profiles showed a fast decline in DR free fraction around DM duration of 20 years, while low‐risk profiles showed a gradual decline. With respect to progression to VTDR, high‐risk profiles risks accumulated gradually after first diagnosis of DR to virtually all affected after >15 years; low‐risk profiles only progressed to VTDR in 50% of cases.
Table 2.
Hazard of developing DR for known risk factors from multivariate Cox regression analysis
| HR | 95% CI | p‐value | |
|---|---|---|---|
| Age of onset T1DM (years) | 1.024 | 1.013–1.035 | <0.001 |
| Mean HbA1c (mmol/mol) | 1.023 | 1.014–1.033 | <0.001 |
| HbA1c variability (CV) | 1.054 | 1.028–1.081 | <0.001 |
| HDL cholesterol (mmol/l) | 0.502 | 0.325–0.775 | 0.002 |
| Total cholesterol (mmol/l) | 1.210 | 1.020–1.436 | 0.029 |
HR = hazard ratio; 95% CI = 95% confidence interval; CV = coefficient of variation; HDL = high‐density lipoprotein.
The hazard ratio for continuous variables should be interpreted as hazard per point of increase in the variable per time unit. For example,: when the HbA1c level increases with 10 points, the instantaneous risk of DR development is 1.02510 = 1.280 or 28.0% higher.
Table 3.
Hazard of developing VTDR for known risk factors from multivariate Cox regression analysis
| HR | 95% CI | p‐value | |
|---|---|---|---|
| Mean HbA1c (mmol/mol) | 1.023 | 1.011–1.036 | <0.001 |
| Albuminuria | 2.940 | 1.130–7.648 | 0.028 |
HR = hazard ratio; 95% CI = 95% confidence interval.
The hazard ratio for continuous variables should be interpreted as hazard per point of increase in the variable per time unit. For example, when the HbA1c level increases with 10 points, the instantaneous risk of DR progression is 1.02410 = 1.268 or 26.8% higher.
Figure 2.
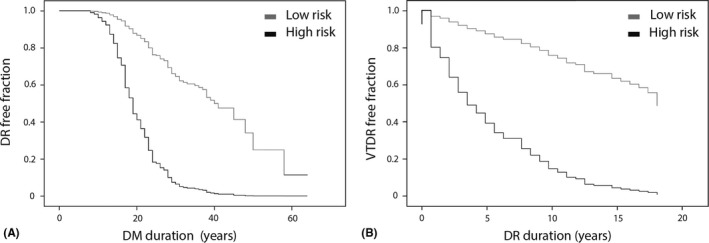
Disease free fraction for low‐ versus high‐risk profiles in: (A) development of DR; (B) progression to VTDR. DR = diabetic retinopathy; DM = diabetes mellitus; VTDR = vision‐threatening diabetic retinopathy.
Risk scores for the study population were calculated and plotted in a histogram (Fig. S1). Although those developing DR/VTDR and those who did not showed somewhat overlapping risk scores, those with higher risk scores more often developed DR and VTDR.
Discussion
In this single centre cohort of subjects with T1DM, mean HbA1c, HbA1c variability, HDL cholesterol, total cholesterol and age of onset of T1DM were independently associated with the hazard of developing DR. The mean HbA1c and albuminuria were associated with the hazard of progression of DR to VTDR.
The effect of hyperglycaemia on development and progression of DR has been studied thoroughly in previous research, and our study confirms the important contribution of HbA1c (DCCT, 1993; Klein et al. 2008, Yau et al. 2012). HbA1c variability has been investigated to a much lesser extent. Hietala et al. (2013b) and Hermann et al. (2014) also reported HbA1c variability as a risk factor for development of DR independently of average glycaemic control. Kilpatrick (2012) proposed several explanatory mechanisms for this correlation. For example, it is possible that with an increase in HbA1c the risk of microvascular complications rises exponentially; therefore, people that experience more HbA1c variation are exposed to a higher average risk. Another possible explanation is that improving glycaemic control can lead to a short‐term ‘early worsening’ in retinopathy, before a subsequent net improvement in the long term, which is a phenomenon that has been reported by the Diabetes Control and Complications Trial (DCCT, 1998). As a consequence of the fast alterations in glycaemic control, the retina may have insufficient time to recover from the damaging effects of previously high HbA1c during periods where HbA1c is low.
In our study, lower HDL cholesterol and higher total cholesterol levels were associated with an increased hazard of DR development. This effect corroborates some, but not all studies, as clinical evidence for a relation between dyslipidaemia and DR is controversial (Chang & Wu 2013). The potential mechanism of action of the association between lipids and DR is also unclear. It has been hypothesized that lipoproteins leak through the disrupted blood–retinal barrier and have a cytotoxic effect on retinal cells (Yu & Lyons 2013). Others hypothesized that lipoproteins interact with the activation of protein kinase C and advanced glycation end product formation – two pathways involved in DR pathophysiology (Chang & Wu 2013).
We found that a higher age of onset of T1DM was associated with a more rapid development of DR. This confirms earlier reports of associations between increasing age of onset of T1DM and development and progression of DR (Hammes et al. 2011; Hietala et al. 2013a; Forga et al. 2016). We hypothesize that subjects with a higher age of onset of T1DM develop DR faster, because the natural ageing process contributes to retinal degeneration, independently of hyperglycaemia. Various microvascular alterations can be observed in the ageing retina, such as increased vessel leakage and declining retinal pigment epithelium cell integrity (Van Kirk et al. 2011; Wei et al. 2017). Additionally, age‐related inflammatory changes are believed to contribute to the pathogenesis of DR (Xu et al. 2009). This could make patients with an older age of onset of T1DM more vulnerable to development of complications.
The link between advanced DR stages and albuminuria has been well established (Cruickshanks et al. 1993; Klein et al. 2008). However, only few studies have investigated the relationship between albuminuria and progression to VTDR in T1DM patients. Lloyd et al. (1995) reported an association between increased albumin excretion rate and progression to proliferative DR over a 2‐year interval. In contrast, no relationship was found between albuminuria and DR progression in two other studies (Lovestam‐Adrian et al. 2001; Klein et al. 2008). This discrepancy is possibly the result of a different definition of progression, as in the latter two studies DR progression was referred to as an increase in DR severity level. Further research is warranted to investigate the discriminative ability of albuminuria as predictor for imminent VTDR in patients with T1DM.
The 25‐year cumulative incidence of DR development (63%) and progression to VTDR (21%) were relatively low when compared the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) group who found rates for 25‐year cumulative incidences of 97% for DR and 42% for proliferative DR, while Broe et al. reported 16‐year cumulative incidences of 95% for DR and 31% for proliferative DR (Klein et al. 2008; Broe et al. 2014). In our cohort, cumulative incidences for BCVA <0.5 and <0.3 were 3% and 1% respectively. The WESDR group reported a 25‐year incidence of 13% for BCVA ≤0.5. A possible explanation for these disparities may lie in the glycaemic control of the patients in our cohort. The measured mean HbA1c level in our cohort was 68 ± 15 mmol/mol versus 91 ± 22 mmol/mol in the WESDR, and 81 ± 17 mmol/mol in the study by Broe et al. (Klein et al. 2010; Broe et al. 2014).
One of the major strengths of this study was the long duration of follow‐up. The average duration of follow‐up was 29 years, thereby offering good insight in the rates of development and progression of DR. Another strength was the use of five separate HbA1c measurements to define the mean HbA1c. This makes the HbA1c level a more reliable parameter and provides a good reflection of HbA1c variability in the 1‐year period prior to diagnosis of DR or the most recent examination. The present study also has its limitations, besides the drawbacks that generally apply to a retrospective study design. In some instances, information on a single measurement was extrapolated in time, whereas patients might have changed their behaviour over time and the investigated variables may have been subject to fluctuations. Furthermore, the study population in this cohort represents a carefully selected phenotyped tertiary care population that may not fully reflect the general population.
In conclusion, the current study provides an overview of the risk factors that are responsible for development and progression of DR in patients with T1DM in a tertiary referral centre. This adds to the growing body of evidence that for proper glycaemic control we should not only focus on absolute HbA1c levels, but also on the level of HbA1c variation. We therefore advise health care professionals involved in the prevention and early detection of retinopathy in patients with T1DM, to take HbA1c variability into account when optimizing glycaemic control.
Supporting information
Fig. S1. Distribution of risk score for: (A) DR; (B) VTDR.
Table S1. Treatments provided during the study period.
This study was supported by BBMRI (enrichment of cohort with ophthalmological phenotyping) for C.J. Tack and by Stichting Blindenhulp for E.K. de Jong.
References
- American Diabetes Association (2008): Standards of medical care in diabetes–2008. Diabetes Care, 31(Suppl 1): S12–S54. [DOI] [PubMed] [Google Scholar]
- Broe R, Rasmussen ML, Frydkjaer‐Olsen U, Olsen BS, Mortensen HB, Peto T & Grauslund J. (2014): The 16‐year incidence, progression and regression of diabetic retinopathy in a young population‐based Danish cohort with type 1 diabetes mellitus: The Danish cohort of pediatric diabetes 1987 (DCPD1987). Acta Diabetol, 51: 413–420. [DOI] [PubMed] [Google Scholar]
- Chang YC & Wu WC. (2013): Dyslipidemia and diabetic retinopathy. Rev Diabet Stud, 10: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshanks KJ, Ritter LL, Klein R & Moss SE (1993): The association of microalbuminuria with diabetic retinopathy. The Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology, 100: 862–867. [DOI] [PubMed] [Google Scholar]
- DCCT (1993): The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med, 329: 977–986. [DOI] [PubMed] [Google Scholar]
- DCCT (1998): Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol, 116: 874–886. [DOI] [PubMed] [Google Scholar]
- Ding J & Wong TY (2012): Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep, 12: 346–354. [DOI] [PubMed] [Google Scholar]
- European Union . Directive 2006/126/EC of the European Parliament and of the council of 20 December 2006 on driving licences (Recast) (Text with EEA relevance) [Online]. Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1514453423639&uri=CELEX:32006L0126 (Accessed on 28 Dec 2017).
- Feldman BS, Cohen‐Stavi CJ, Leibowitz M, Hoshen MB, Singer SR, Bitterman H, Lieberman N & Balicer RD (2014): Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS ONE, 9: e108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forga L, Goni MJ, Ibanez B, Cambra K, Garcia‐Mouriz M & Iriarte A (2016): Influence of age at diagnosis and time‐dependent risk factors on the development of diabetic retinopathy in patients with type 1 diabetes. J Diabetes Res, 2016: 9898309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Kerner W, Hofer S, Kordonouri O, Raile K & Holl RW (2011): Diabetic retinopathy in type 1 diabetes‐a contemporary analysis of 8,784 patients. Diabetologia, 54: 1977–1984. [DOI] [PubMed] [Google Scholar]
- Hermann JM, Hammes HP, Rami‐Merhar B, Rosenbauer J, Schutt M, Siegel E, Holl RW & Mellitus DPVItGBCND (2014): HbA1c variability as an independent risk factor for diabetic retinopathy in type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS ONE, 9: e91137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietala K, Forsblom C, Summanen P & Groop PH (2013a): Higher age at onset of type 1 diabetes increases risk of macular oedema. Acta Ophthalmol, 91: 709–715. [DOI] [PubMed] [Google Scholar]
- Hietala K, Waden J, Forsblom C, Harjutsalo V, Kyto J, Summanen P, Groop PH, FinnDiane Study G (2013b): HbA1c variability is associated with an increased risk of retinopathy requiring laser treatment in type 1 diabetes. Diabetologia, 56: 737–745. [DOI] [PubMed] [Google Scholar]
- Kilpatrick ES (2012): The rise and fall of HbA(1c) as a risk marker for diabetes complications. Diabetologia, 55: 2089–2091. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Lee KE, Gangnon R & Klein BE (2008): The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty‐five‐year progression of retinopathy in persons with type 1 diabetes. Ophthalmology, 115: 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Lee KE, Gangnon RE & Klein BE (2010): The 25‐year incidence of visual impairment in type 1 diabetes mellitus the Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology, 117: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CE, Klein R, Maser RE, Kuller LH, Becker DJ & Orchard TJ (1995): The progression of retinopathy over 2 years: the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. J Diabetes Complications, 9: 140–148. [DOI] [PubMed] [Google Scholar]
- Lovestam‐Adrian M, Agardh CD, Torffvit O & Agardh E (2001): Diabetic retinopathy, visual acuity, and medical risk indicators: a continuous 10‐year follow‐up study in Type 1 diabetic patients under routine care. J Diabetes Complications, 15: 287–294. [DOI] [PubMed] [Google Scholar]
- Luk AO, Ma RC, Lau ES et al. (2013): Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev, 29: 384–390. [DOI] [PubMed] [Google Scholar]
- Miljanovic B, Glynn RJ, Nathan DM, Manson JE & Schaumberg DA (2004): A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes, 53: 2883–2892. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, NH, Lung, and Blood Institute (2001). Third report of the National Cholesterol Education Program (NCEP) on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. NIH Publication No. 02‐5215. Bethesda: NIH.
- Raymond NT, Varadhan L, Reynold DR et al. (2009): Higher prevalence of retinopathy in diabetic patients of South Asian ethnicity compared with white Europeans in the community: a cross‐sectional study. Diabetes Care, 32: 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejling AS, Schouwenberg B, Faerch LH, Thorsteinsson B, de Galan BE & Pedersen‐Bjergaard U (2016): Association between hypoglycaemia and impaired hypoglycaemia awareness and mortality in people with Type 1 diabetes mellitus. Diabet Med, 33: 77–83. [DOI] [PubMed] [Google Scholar]
- Van Kirk CA, VanGuilder HD, Young M, Farley JA, Sonntag WE & Freeman WM (2011): Age‐related alterations in retinal neurovascular and inflammatory transcripts. Mol Vis, 17: 1261–1274. [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Jiang H, Shi Y, Qu D, Gregori G, Zheng F, Rundek T & Wang J (2017): Age‐related alterations in the retinal microvasculature, microcirculation, and microstructure. Invest Ophthalmol Vis Sci, 58: 3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2015): International Classification of Diseases and Health Related Problems 10th Revision (ICD‐10).
- Wilkinson CP, Ferris FL 3rd, Klein RE et al. (2003): Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology, 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M & Forrester JV (2009): Para‐inflammation in the aging retina. Prog Retin Eye Res, 28: 348–368. [DOI] [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R et al. (2012): Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care, 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY & Lyons TJ (2013): Modified Lipoproteins in Diabetic Retinopathy: a Local Action in the Retina. J Clin Exp Ophthalmol, 4: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Distribution of risk score for: (A) DR; (B) VTDR.
Table S1. Treatments provided during the study period.


