Abstract
A population pharmacokinetic analysis was conducted from a subset of samples obtained from the Lung Function and Quality of Life Assessment in Chronic Obstructive Pulmonary Disease with Closed Triple Therapy trial to characterize the pharmacokinetics of fluticasone furoate, umeclidinium, and vilanterol in patients with symptomatic COPD following treatment with fluticason furoate‐umeclidinium‐vilanterol combined in a single inhaler. This was a randomized, double‐blind, double‐dummy study comparing 24 weeks of once‐daily triple therapy (fluticason furoate‐umeclidinium‐vilanterol, 100 μg/62.5 μg/25 μg; Ellipta inhaler) with twice‐daily dual therapy (budesonide/formoterol 400 μg/12 μg; Turbuhaler). The analyses were conducted in a subset of 74 patients who received fluticason furoate‐umeclidinium‐vilanterol and provided serial or sparse samples. Monte Carlo simulations and a model‐based estimation approach both indicated that systemic drug concentrations of fluticasone furoate, umeclidinium, and vilanterol after administration of fluticason furoate‐umeclidinium‐vilanterol triple combination therapy from a single inhaler were within the ranges observed following administration of these drugs as monotherapy (fluticasone furoate, umeclidinium, and vilanterol) or as dual‐combination therapy (fluticasone furoate/vilanterol or umeclidinium/vilanterol).
Keywords: COPD, single‐inhaler triple therapy, fluticasone furoate, umeclidinium, vilanterol
Treatment of chronic obstructive pulmonary disease (COPD) commonly includes triple therapy with one or more long‐acting bronchodilators (long‐acting muscarinic receptor antagonists [LAMA]), long‐acting beta2‐adrenergic receptor agonists [LABA]) and inhaled corticosteroids (ICS) for patients with more advanced disease who have significant symptoms and a high risk for exacerbations.1 Studies have shown that the use of ICS/LAMA/LABA delivered via multiple inhalers in patients with moderate to severe COPD demonstrates greater improvements in lung function and health‐related quality of life compared with ICS/LABA or LAMA therapy alone.2, 3
Recently, a once‐daily single‐inhaler triple therapy of fluticasone furoate, umeclidinium, and vilanterol (fluticason furoate‐umeclidinium‐vilanterol) 100 μg/62.5 μg/25 μg was approved in the United States for the treatment of COPD. The Lung Function and Quality of Life Assessment in COPD with Closed Triple Therapy (FULFIL) trial was the first study to compare once‐daily single‐inhaler triple therapy (ICS/LAMA/LABA) with twice‐daily dual therapy (ICS/LABA) in patients with symptomatic COPD who were at risk for exacerbations.4 The FULFIL study demonstrated statistically significant and clinically meaningful improvements at 24 weeks of therapy in lung function and health‐related quality of life with once‐daily single‐inhaler triple therapy delivered using the Ellipta inhaler (GlaxoSmithKline, Research Triangle Park, North Carolina), compared with twice‐daily budesonide/formoterol (400/12 μg) delivered using the Turbuhaler (AstraZeneca, Wilmington, Delaware). The study also demonstrated a significant reduction in the annual rate of moderate/severe exacerbations with fluticason furoate‐umeclidinium‐vilanterol compared with budesonide/formoterol. The safety profile of the fluticason furoate‐umeclidinium‐vilanterol single‐inhaler triple therapy was similar to that of the individual components, with no new safety findings.
The pharmacokinetics (PK) of fluticasone furoate, umeclidinium, and vilanterol have been described previously in monotherapy or dual combination therapy (fluticasone furoate/vilanterol or umeclidinium/vilanterol) studies.5, 6 In healthy volunteer studies, there was no evidence of a pharmacokinetic or pharmacodynamic interaction when the 3 molecules were combined in 1 device compared with dual therapies.7 The current report provides the first analysis of the population PK of fluticasone furoate, umeclidinium, and vilanterol when administered as a fixed‐dose combination from a single inhaler.
Methods
Study Design, Treatment, and Patient Eligibility
A total of 162 centers in 15 countries randomized and treated subjects: 21 centers in Russian Federation, 17 in Ukraine, 17 in Mexico, 16 in Germany, 12 in Greece, 10 in Czech Republic, 10 in Romania, 9 in Bulgaria, 8 in China, 8 in Estonia, 8 in Hungary, 8 in Italy, 6 in Poland, 6 in Republic of Korea, and 6 in Slovakia. The study was carried out according to the Declaration of Helsinki, good clinical practice guidelines of the International Conference on Harmonization, and other applicable regulatory requirements. The study protocol was approved by institutional review boards for human studies associated with the clinical sites and written consent was obtained from each patient or their surrogates prior to study participation. A complete list of all study sites and details of their respective institutional review boards is provided as Supplemental Data.
The complete design of the FULFIL study was previously reported.4 In brief, eligible patients were aged ≥40 years with COPD (forced expiratory volume in 1 second [FEV1] <50% and COPD Assessment Test [CAT™; GlaxoSmithKline, Research Triangle Park, North Carolina] score ≥10, or FEV1 ≥50‐<80% and CAT score ≥10, and either ≥2 moderate exacerbations in the past year or ≥1 severe exacerbation in the past year). Patients had to have received daily maintenance COPD therapy for ≥3 months prior to screening. Patients were randomly assigned 1:1 to receive 24 weeks of treatment with once‐daily fluticason furoate‐umeclidinium‐vilanterol or twice‐daily budesonide/formoterol; a subset of patients received blinded study treatment for up to 52 weeks.
Pharmacokinetic Assessments
Blood samples were collected from a preplanned subset of approximately 130 subjects for the sparse sampling scheme and approximately 20 subjects for the serial sampling scheme in order to achieve an adequate number of samples from subjects randomly assigned to the fluticason furoate‐umeclidinium‐vilanterol arm. Blood samples at weeks 12 and 24 were analyzed from 74 patients who had been randomly assigned to the fluticason furoate‐umeclidinium‐vilanterol arm (fluticason furoate‐umeclidinium‐vilanterol PK population). The sampling scheme included 2 mutually exclusive subsets of subjects (sparse and serial subsets) that provided PK blood samples. For the sparse sampling, two 6‐mL samples (n = 64) were collected at week 12 (prior to dosing and 5–15 minutes after dosing) and week 24 (5–15 minutes and 45–90 minutes after dosing). For the serial sampling, seven 6‐mL samples were collected at week 24 (prior to dosing and 5–15 minutes, 45–90 minutes, 2.5–4 hours, 6–8 hours, 10–12 hours, and 23–24 hours after dosing).
Plasma samples were analyzed for fluticasone furoate, umeclidinium, and vilanterol concentrations using validated bioanalytical methods based on solid phase extraction followed by high‐pressure liquid chromatography with tandem mass spectrometry for analyte detection. The lower limit of quantification for fluticasone furoate, umeclidinium, and vilanterol was 10 pg/mL; the higher limit of quantification was 1000 pg/mL for fluticasone furoate and vilanterol and 2000 pg/mL for umeclidinium.
Population Pharmacokinetic Analysis
Population PK analyses used nonlinear mixed effects modeling with NONMEM program version 7.1.2 (GloboMax, Hanover, Maryland) for modeling and simulations.
Because FULFIL (CTT116853) data are within the prediction limits of the existing models based on extensive clinical pharmacology data (including population PK) from the mono‐ (umeclidinium) and dual‐therapy programs (fluticasone furoate/vilanterol, umeclidinium/vilanterol), a covariate analysis was not planned. The same covariate relationship was assumed and consistent parameter estimates between the current and previous model supported that assumption (Tables 1 and 2).
Table 1.
Final fluticasone furoate Pharmacokinetic Model: PK Parameter Estimates
| Combined Model Ln Estimates | Historical Model Ln Estimates | Model Parameter Estimates With Combined Dataset | Historical Model Parameter Estimates | |
|---|---|---|---|---|
| Parameter | (95%CI) | (95%CI) | (95%CI) | (95%CI) |
| CL/F (L/h) | 5.43 | 5.44 | 228 | 230 |
| (5.38 to 5.48) | (5.39 to 5.49) | (217 to 240) | (219 to 242) | |
| V2/F (L) | 0.31 | 0.31 | 1.36 | 1.36 |
| (Fixed) | (Fixed) | (Fixed) | (Fixed) | |
| Q/F (L/h) | 5.74 | 5.59 | 311 | 268 |
| (5.54 to 5.94) | (5.40 to 5.78) | (255 to 380) | (221 to 324) | |
| V3 /F (L) | 4.66 | 4.71 | 106 | 111 |
| (4.45 to 4.87) | (4.51 to 4.91) | (86 to 130) | (90.9 to 136) | |
| KA (h‐1) | –2.94 | –2.95 | 0.053 | 0.052 |
| (–3 to 2.88) | (–3.01 to 2.89) | (0.049 to 0.056) | (0.049 to 0.056) |
CL/F, inhaled clearance; V2/F, volume of central compartment; Q/F, intercompartmental clearance; V3/F, volume of peripheral compartment; KA, absorption rate; CI, confidence interval; Ln, Log transformed.
Table 2.
Final umeclidinium and vilanterol Pharmacokinetic Model: PK Parameter Estimates
| Parameter | Model Parameter Estimates With Combined Dataset (RSE%) | Historical Model Parameter Estimates (RSE%) |
|---|---|---|
| umeclidinium | ||
| CL/F (L/h) | 210 (2.9) | 218 (2.3) |
| V2/F (L) | 1170 (1.12) | 1160 (2.8) |
| Q/F (L/h) | 854 (5.4) | 873 (4.7) |
| V3 /F (L) | 16200 (7.28) | 30200 (22.1) |
| KA (h‐1) | 40.3 (300) | 39.1 (43.7) |
| vilanterol | ||
| CL/F (L/h) | 41.6 (1.5) | 40.9 (1.4) |
| V2/F (L) | 271 (2.1) | 268 (2.0) |
| Q/F (L/h) | 116 (4.4) | 118 (4.6) |
| V3 /F (L) | 1280 (4.6) | 1240 (6.3) |
| KA (h‐1) | 19.6 (9.5) | 18.8 (12.8) |
CL/F, inhaled clearance; V2/F, volume of central compartment; Q/F, intercompartmental clearance; V3/F, volume of peripheral compartment; KA, absorption rate; CI, confidence interval.
Data were analyzed using 2 different approaches in this study. In the first approach, Monte Carlo simulations of previously reported population PK models for fluticasone furoate, umeclidinium, and vilanterol in patients with COPD who received fluticasone furoate, umeclidinium, and vilanterol as monotherapy or as dual combination therapy (fluticasone furoate/vilanterol or umeclidinium/vilanterol) were undertaken.5, 6 Specifically, the final PK model for fluticasone furoate was a 2‐compartment model with first‐order absorption and first‐order elimination rate, with covariate effect of “race” on apparent clearance (CL/F, inhaled). A 3‐compartment linear model with zero‐order absorption and first‐order elimination was the model used for vilanterol, with covariates of effect of age (on CL/F and V1/F), body weight (on CL/F), sex and smoking (on V1/F). A 2‐compartment model with first‐order absorption was the final model for umeclidinium, with body weight, age, and creatinine clearance covariate effect on CL/F of umeclidinium. NONMEM codes are provided for the 3 models as supplementary tables.
The observed time versus plasma concentration data for each analyte from the current study, FULFIL, were overlaid on the 90% prediction intervals from the respective simulations to gauge adequacy of these existing models to describe the observed data. The maximum a posteriori (MAP) Bayesian estimates of the PK parameters were obtained for all 74 patients, which were used to compute steady‐state maximum plasma drug concentration (Cmax) and area under the plasma drug concentration‐time curve (AUC) over the dosing interval.
In the second approach, a model‐based estimation was applied. The plasma data for fluticasone furoate, umeclidinium, and vilanterol from FULFIL were combined with the respective historical data used to develop population PK models for fluticasone furoate, umeclidinium, and vilanterol and were analysed using previously reported population PK models for fluticasone furoate, umeclidinium, and vilanterol.5, 6 The data below the quantifiable limit (BQL) were treated as censored data and analyzed using a joint probability model that maximizes the likelihood of data above quantification limit and treats BQL data as censored with the full likelihood approach.8 The MAP Bayes PK parameter estimates were compared following analysis of the combined dataset (historical data set, including the FULFIL data) versus the original historical data (without the FULFIL data).
Results
The demographics and baseline characteristics of patients included in the population PK analysis were similar to the intent‐to‐treat population of the FULFIL study (Table 3).
Table 3.
Summary of Patient Demographics
| PK Population | ITT Population | |
|---|---|---|
| (n = 74) | (N = 1810) | |
| Mean age, years (SD) | 64 (6.5) | 64 (8.6) |
| Sex, n (%) | ||
| Female | 19 (26) | 469 (26) |
| Male | 55 (74) | 1341 (74) |
| Mean BMI, kg/m2 (SD) | 28 (4.2) | 27 (5.1) |
| Mean height, cm (SD) | 171 (9.3) | 170 (8.8) |
| Mean weight, kg (SD) | 81 (16) | 78 (16.8) |
| White, n (%) | 74 (100) | 1543 (85) |
| Mean % predicted FEV1 (SD) | 45 (15) | 42 (13.3) |
As shown in Figure 1, the observed plasma concentration‐time data for fluticasone furoate, umeclidinium, and vilanterol in FULFIL were all within the range observed for historical fluticasone furoate, umeclidinium, and vilanterol data, respectively. In addition, the observed plasma concentration‐time data for all 3 analytes (fluticasone furoate, umeclidinium, and vilanterol) in FULFIL were generally contained within the 90% prediction intervals, as indicated by the 5% and 95% percentiles obtained from the Monte Carlo simulations of the population PK models for fluticasone furoate, umeclidinium, and vilanterol from previous models, with fluticasone furoate/vilanterol and umeclidinium/vilanterol suggesting the adequacy of existing population PK models (Figure 2). The model also considered censored data and adequately characterized the proportion of BQL data for each drug in this population (Figure 3). This was further confirmed using the model‐based estimation approach in which there were no marked changes in the PK parameter estimates of fluticasone furoate, umeclidinium, and vilanterol using the combined dataset (FULFIL and historical data) versus the historical data (without data from FULFIL) (Tables 1 and 2).
Figure 1.
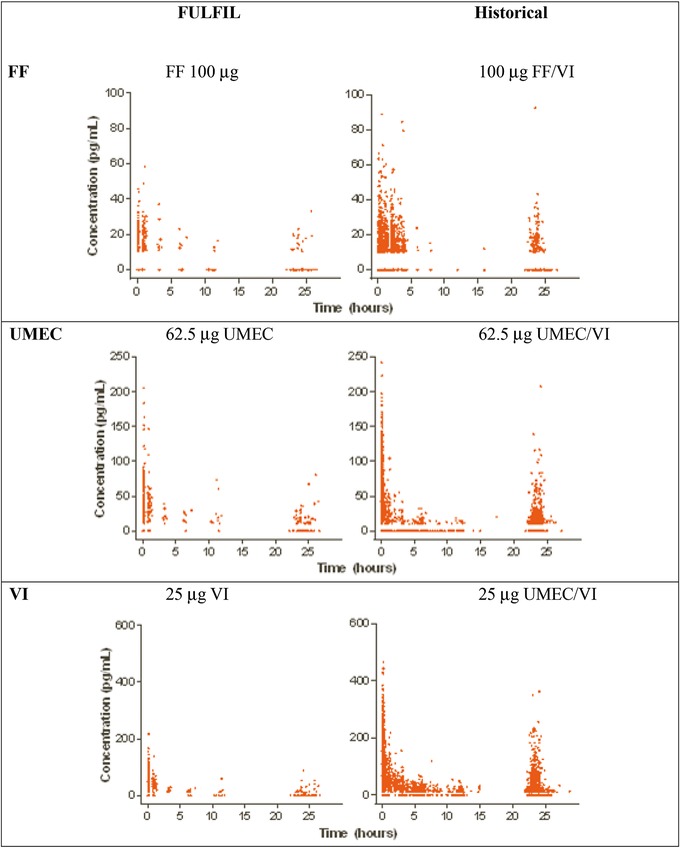
Observed concentration‐time data from FULFIL and historical datasets. FF: Fluticasone furoate; UMEC: umeclidinium; VI: vilanterol.
Figure 2.
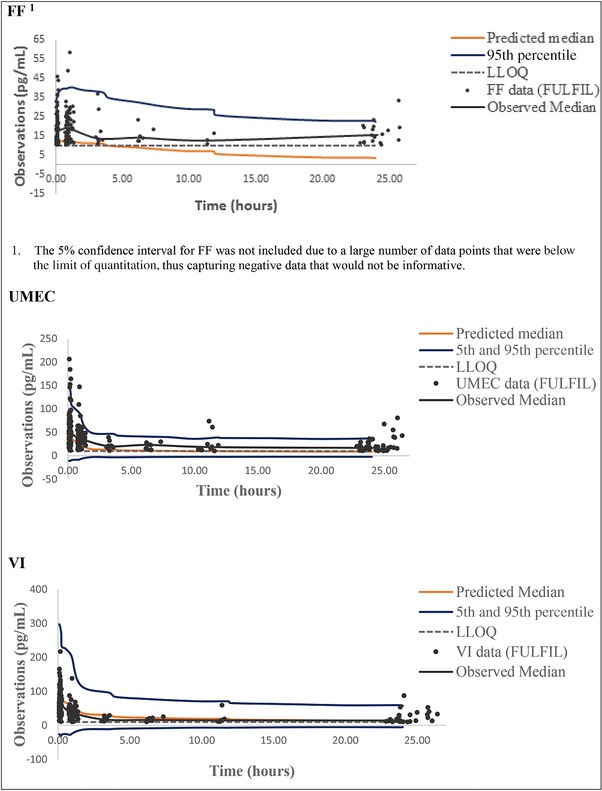
Comparison of observed versus predicted concentration‐time data profiles. FF: Fluticasone furoate; UMEC: umeclidinium; VI: vilanterol.
Figure 3.
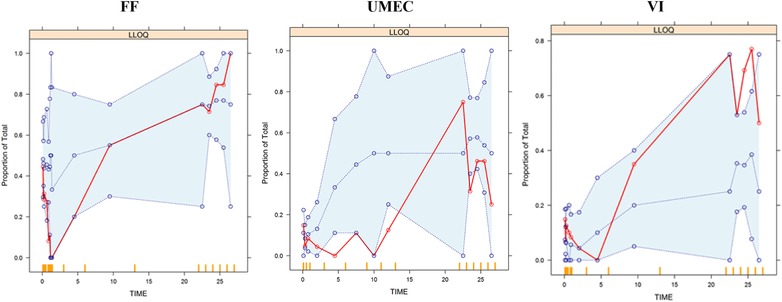
Proportion of values data below the quantifiable limit (BQL). Solid lines = observed intervals; dashed lines = prediction intervals. FF: Fluticasone furoate; UMEC: umeclidinium; VI: vilanterol.
Based on the individual estimated MAP Bayes PK parameter estimates for fluticasone furoate, umeclidinium, and vilanterol administered as the triple fixed‐dose combination (fluticason furoate‐umeclidinium‐vilanterol), steady‐state systemic exposures were computed and are summarized in Table 4. The steady‐state AUC over dosing interval for fluticasone furoate, umeclidinium, and vilanterol estimated from the population PK analysis in the FULFIL study (with fluticason furoate‐umeclidinium‐vilanterol) was consistent with historical data in patients with COPD given mono‐ or dual‐combination therapies. The Cmax at steady state for fluticasone furoate following fluticason furoate‐umeclidinium‐vilanterol was also consistent with historical data. Corresponding Cmax values for umeclidinium and vilanterol were generally lower with fluticason furoate‐umeclidinium‐vilanterol (likely due to inadequate characterization of time to peak plasma concentration in a small number of subjects) compared with historical data from dual combinations. The lower Cmax for umeclidinium and vilanterol was not expected to be clinically relevant with respect to safety or efficacy following administration of the single‐inhaler triple fluticason furoate‐umeclidinium‐vilanterol combination, as observed in the overall analysis of the FULFIL study.3
Table 4.
Summary of Steady‐State Systemic Cmax and AUC Results for fluticasone furoate, umeclidinium, and vilanterol from FULFIL and Historic Data
| Geometric Mean | Geometric Mean | |||
|---|---|---|---|---|
| Cmax, pg/mL | AUC(0‐24h), pg‐h/mL | |||
| Study | Treatment (μg) | n | (95%CI) | (95%CI) |
| Fluticasone furoate | ||||
| FULFIL | Fluticason furoate‐umeclidinium‐vilanterol 100/62.5/25 | 74 | 13.2 (11.2‐15.1) | 188 (160‐216) |
|
Fluticasone furoate/vilanterol 100/25 | 391 | 11.9 (10.9‐12.9) | 182 (170‐195) |
|
Fluticasone furoate 100 | 333 | 11.5 (10.5‐12.4) | 181 (167‐196) |
| umeclidinium | ||||
| FULFIL | Fluticason furoate‐umeclidinium‐vilanterol 100/62.5/25 | 74 | 55.7 (50.4‐60.9) | 341 (301‐381) |
| DB2113373 | Umeclidinium/vilanterol 62.5/25 | 410 | 68.5 (65.2‐71.9) | 308 (293‐328) |
| DB2113373 | Umeclidinium 62.5 | 417 | 70.3 (67.0‐73.8) | 318 (303‐334) |
| DB2113373 | Mono and combo 62.5, 62.5/25 | 827 | 69.3 (67.0‐71.6) | 312 (302‐323) |
| Vilanterol | ||||
| FULFIL | Fluticason furoate‐umeclidinium‐vilanterol 100/62.5/25 | 74 | 101.4 (91.1‐111.9) | 666 (604‐728) |
| DB2113373 | Umeclidinium/vilanterol 62.5/25 | 410 | 128.2 (122.1‐134.6) | 612 (589‐637) |
| DB2113373 | Vilanterol 25 | 421 | 128.2 (122.0‐134.6) | 613 (589‐637) |
| DB2113361 | Umeclidinium/vilanterol 125/25 | 402 | 128.4 (122.3‐135.0) | 617 (592‐642) |
| DB2113361 | Vilanterol 25 | 404 | 128.2 (122.0‐134.9) | 611 (587‐635) |
|
All arms combined | 1637 | 127.9 (124.9‐131.0) | 615 (603‐627) |
The steady‐state estimates were calculated using individual maximum a posteriori (MAP) Bayesian estimates.
Discussion
FULFIL is the first study providing data on the PK of fluticasone furoate, umeclidinium, and vilanterol in patients with COPD when administered as fluticason furoate‐umeclidinium‐vilanterol in combination using a single inhaler. This report describes the population pharmacokinetic PK analysis of data from the subset of patients who participated in the FULFIL study.
The population PK design following fluticason furoate‐umeclidinium‐vilanterol dosing in this study focused on the initial characterization of the systemic exposure (rate and extent) of the individual analytes fluticasone furoate, umeclidinium, and vilanterol in a cohort of patients with symptomatic COPD. As mentioned above, a descriptive population PK analysis was undertaken in the current study, without any covariate analysis, by leveraging the availability of extensive clinical pharmacology data (including population PK models) for fluticasone furoate, umeclidinium, and vilanterol from previous mono‐ and dual‐therapy programs.
In the FULFIL study, consistency of the predicted PK parameter estimates using the model‐based simulation and estimation approaches suggested no major differences in the patient population characteristics in the FULFIL study in relation to the historical COPD population studied with fluticasone furoate, umeclidinium, and vilanterol as mono‐ and dual therapies. In addition, simulation‐based diagnostics demonstrated that the population PK models adequately predicted the fluticasone furoate, umeclidinium, and vilanterol PK data from the FULFIL study. The model also considered censored data and adequately characterized the proportion of BQL data for each drug in this population. The model‐based estimates of Cmax and AUC values at steady‐state from FULFIL were within the range observed in historical studies involving fluticasone furoate, umeclidinium, and vilanterol administered in subjects with COPD. Previous data from healthy volunteers also confirmed the absence of a relevant pharmacokinetic/pharmacodynamic interaction when these 3 molecules were combined.7
The extensive clinical pharmacology programs for mono‐ and dual‐therapy combinations did not demonstrate any PK interactions between fluticasone furoate, umeclidinium, and vilanterol. The population PK analysis in the present study provided reasonable estimates of rate and extent of systemic exposure to fluticasone furoate, umeclidinium, and vilanterol in individuals with symptomatic COPD. The FULFIL population PK analyses showed that systemic drug levels of fluticasone furoate, umeclidinium, and vilanterol following fluticason furoate‐umeclidinium‐vilanterol administration in one inhaler (single‐inhaler triple combination) were within the range observed with administration through individual single inhalers (fluticasone furoate, umeclidinium, and vilanterol).
Supporting information
Supporting Information
Author Contributions
All authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. GSK participated in all aspects of this study, including design, conduct, data collection, data management, data analysis, data interpretation, as well as the preparation, review, and approval of the manuscript. RM, DL, and NB conceived and designed the study. DL, HB, NB, and RB were involved with the oversight of the clinical study. RM, DL, NB, CQZ, and EP were involved with data analysis. All authors contributed to the manuscript.
Disclosures
Funding for this study CTT116853 (NCT02345161) was provided by GlaxoSmithKline. RM, EP, MB, NB, RB, CQZ, and DL are employees of GSK and hold stock in the company. HB was an employee of GSK at the time of the study conduct and has since retired. Editorial support (development of the first draft, assembling tables and figures, collating author comments, and referencing) was provided by Guissou Dabiri, PhD, of GD Scientific & Medical Writing, LLC, and was funded by GSK.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD) . GOLD 2017 global strategy for the diagnosis, management and prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/.
- 2. Kerwin EM, Scott‐Wilson C, Sanford L, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPD. Respir Med. 2013;107(4):560–569. [DOI] [PubMed] [Google Scholar]
- 3. Martinez FJ, Boscia J, Feldman G, et al. Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trial. Respir Med. 2013;107(4):550–559. [DOI] [PubMed] [Google Scholar]
- 4. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once‐daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:438–446. [DOI] [PubMed] [Google Scholar]
- 5. Goyal N, Beerahee M, Kalberg C, Church A, Kilbride S, Mehta R. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet. 2014;53:637–648 [DOI] [PubMed] [Google Scholar]
- 6. Siederer S, Allen A, Yang S, Population pharmacokinetics of inhaled fluticasone furoate and vilanterol in subjects with chronic obstructive pulmonary disease. Eur J Drug Metab Pharmacokinet. 2016;41:743–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brealey N, Gupta A, Renaux J, et al. Pharmacokinetics of fluticasone furoate, umeclidinium, and vilanterol as a triple therapy in healthy volunteers. Int J Clin Pharmacol Ther. 2015;53:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM vilanterol. J Pharmacokinet Pharmacodyn. 2008;35:401–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


