Figure 3.
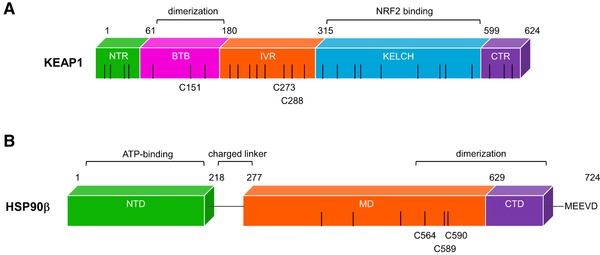
Schematic representation of mouse KEAP1 and human HSP90β. A) KEAP1 is a homodimeric protein which has five distinct domains: N‐terminal region (NTR), broad complex, Tramtrack, Bric‐á‐brac (BTB), intervening region (IVR), Kelch domain (KELCH), and the C‐terminal region (CTR). B) HSP90β has an N‐terminal domain (NTD), where ATP binds. The middle domain (MD) allows for client protein binding and the C‐terminal part of the MD together with the C‐terminal domain (CTD) allows for homodimerization of the chaperone. Various co‐chaperones are able to bind to all three domains with different affinities. Client proteins are also able to interact with each of the HSP90 domains. The black bars represent cysteine residues present in each of the proteins, and some of the reactive cysteines are indicated.
