Abstract
Invasion and migration is the hallmark of malignant tumors as well as the major cause for breast cancer death. The polypyrimidine tract binding, PTB, protein serves as an important model for understanding how RNA binding proteins affect proliferation and invasion and how changes in the expression of these proteins can control complex programs of tumorigenesis. We have investigated some roles of polypyrimidine tract binding protein 1 (PTBP1) in human breast cancer. We found that PTBP1 was upregulated in breast cancer tissues compared with normal tissues and the same result was confirmed in breast cancer cell lines. Knockdown of PTBP1 substantially inhibited tumor cell growth, migration, and invasion. These results suggest that PTBP1 is associated with breast tumorigenesis and appears to be required for tumor cell growth and maintenance of metastasis. We further analyzed the relationship between PTBP1 and clinicopathological parameters and found that PTBP1 was correlated with her‐2 expression, lymph node metastasis, and pathological stage. This will be a novel target for her‐2(+) breast cancer. PTBP1 exerts these effects, in part, by regulating the phosphatase and tensin homolog‐phosphatidylinositol‐4,5‐bisphosphate 3‐kinase/protein kinase B (PTEN‐PI3K/Akt) pathway and autophagy, and consequently alters cell growth and contributes to the invasion and metastasis.
Keywords: autophagy, breast cancer, polypyrimidine tract binding protein 1 (PTBP1), PTEN/Akt signal pathway
1. INTRODUCTION
Breast cancer is the most common cancer and the leading cause of cancer‐associated mortality in women worldwide (Chen et al., 2016). Despite years of intensive study and substantial progress, our understanding of the mechanisms that mediate the development and progression of breast cancer is still incomplete. The abnormal proliferation and migration of tumor cells is a hallmark of tumor pathology during tumor progression.
The polypyrimidine tract binding protein, PTB, was among the first to be discovered as a regulator of RNA splicing (Wollerton, Gooding, Wagner, Garcia‐Blanco, & Smith, 2004). Cancer‐specific splicing events have been reported at the messenger ribonucleic acid (mRNA) level in colon, bladder, and prostate cancers (Thorsen et al., 2008). Growing evidence has shown that PTB makes great contributions to malignant transformation, since it may be particularly relevant in the etiology of cancer, provide selective drug targets, or serve as a marker set for cancer diagnosis (Pesson et al., 2014; Venables, 2006; Zhang & Manley, 2013). We found that PTB protein 1 (PTBP1) is an important factor in maintaining breast cancer cell growth and malignant properties. The PTB protein, also known as PTBP1 or hnRNP1, is one of the most investigated RNA‐binding proteins (RBP) in vertebrates. It is a key factor in the control of RNA metabolism, as it was shown to regulate mRNA alternative splicing events, mRNA stability, mRNA localization, and internal ribosome entry site dependent translation (Llorian et al., 2010; Sauliere, Sureau, Expert‐Bezancon, & Marie, 2006; Xue et al., 2009). PTBP1 also has a role in determining mRNA localization in the cytoplasm (Cote et al., 1999). Another important function of PTBP1 is its involvement in internal ribosome entry site mediated translation (Auweter & Allain, 2008).
It has been found that PTBP1 is overexpressed in human epithelial ovarian tumors (He, Ee, Coon, & Beck, 2004; He et al., 2007) and glioblastomas compared with normal tissues. Knockdown of PTBP1 expression in tumor cell lines, such as A2780 (ovarian cancer) and PC‐3M (prostate cancer), significantly impaired growth of tumor cells and their malignant properties (Jin, McCutcheon, Fuller, Huang, & Cote, 2000; Wang et al., 2008). The expression levels of PTBP1 have been found to be elevated in brain tumors (Cheung et al., 2009; McCutcheon, Hentschel, Fuller, Jin, & Cote, 2004), ovarian tumors (He et al., 2007), and different malignant cell lines (Wang et al., 2008). Furthermore, high expression of PTBP1 has been found to be associated with the aggressive behavior of several types of cancer, especially in glioma and ovarian tumors (Cheung et al., 2009; He et al., 2007).
In our study, we investigated the role of PTBP1 in breast cancer. We found that PTBP1 was overexpressed in breast cancer tissues compared with normal tissues and breast cancer cell lines, which was accompanied by increased p‐Akt and decreased phosphatase and tensin homolog (PTEN) expression. Meanwhile, we found that PTBP1 inducing the transition of the LC3BII to LC3BI is a significant event in autophagy. Knockdown of PTBP1 expression substantially decreased tumor cell proliferation, migration, and invasion. Knockdown of PTBP1 inhibited breast cancer cell growth in nude mice. We further analyzed the relationship between PTBP1 and clinicopathological parameters. The analyzed parameters were tumor size, axillary lymph node metastasis (pN), estrogen receptor (ER), progesterone receptor (PR), pathological stage, and her‐2. We found that PTBP1 was correlated with her‐2 expression, lymph node metastasis, and pathological stage. Together, these results suggest that PTBP1 is involved in breast tumorigenesis and maybe a new target for breast cancer therapy.
2. MATERIALS AND METHODS
2.1. Clinical samples and mice
One hundred and thirty seven paired breast cancer tissues and adjacent normal tissues/paracancerous normal tissues (N 5 cm away from the tumor edge) were obtained/collected from the Department of Breast Surgery of the First Affiliated Hospital of the China Medical University. All of these patients had a clear/definite histological diagnosis of breast cancer based on/according to the American Joint Committee Cancer (AJCC). Fresh tumor and adjacent noncancerous tissues were harvested after resection and then immediately frozen in liquid nitrogen and kept/conserved at − 80°C. The study was approved by the Medical Ethics and Human Clinical Trial Committee of the China Medical University. Five to six weeks old female BALB/c nude mice were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). All animal experiments were conducted in accordance with/on the basis of the Guide for the Care and Use of Laboratory Animals (NIH publication no. 80–23, revised 1996) and approved by the Animal Ethic Committee of the China Medical University.
2.2. Cell culture
Normal human breast epithelial cell line MCF‐10A was cultured in Mammary Epithelial Cell Growth Medium (Lonza No. CC‐3150) with 100 ng/ml cholera toxin (2‐mg vials; Sigma No. C‐8052, Grand Island). Human breast cancer cell lines ZR‐75‐1, ZR‐75‐30, Hs578T were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) containing 10% fetal bovine serum (FBS). Human breast cancer cell line MCF‐7 was cultured in modified Eagle's medium (Gibco) containing 10% FBS and 10 mg/l insulin. Human breast cancer cell line SK‐BR‐3 was cultured in McCoy’s 5a modified medium containing 10% FBS. Human breast cancer cell lines MDA‐MB‐453 and MDA‐MB‐231 were cultured in Leibovitz’s L‐15 containing 10% FBS. Human breast cancer cell line HCC1937 was cultured in Roswell Park Memorial Institute‐1640 (RPMI‐1640) medium containing 10% FBS. The cells were incubated at 37°C in a humidified incubator containing 5% CO2.
2.3. Western blot assay
Cells and tissues were lysed in a radio immunoprecipitation assay (RIPA) lysis buffer supplemented with protease inhibitors (Roche). Total protein extracts were separated by 8% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and then transferred to a polyvinylidenefluoride (PVDF) membrane (Millipore). The membranes were blocked in 5% skimmed milk for 1 hr and incubated with anti‐PTBP1 (1:1,000; Abcam), anti‐PTEN (1:1,000; Abcam), anti‐Akt (1:1,000; Abcam), anti‐p‐Akt (1:1,000; Abcam), anti‐LC3B (1:1,000; Abcam), anti‐Flag (1:2,000; ShangHai Ruixing), anti‐Actin (1:5,000; Abcam, as a loading control), and anti‐glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (1:15,000; KangChen, China, as a loading control) antibodies. Appropriate primary antibodies were added at 4°C overnight. Appropriate secondary antimouse and antirabbit antibodies were added for 2 hr at room temperature. Finally, for detection of the proteins, an enhanced chemiluminescence (ECL) chemiluminescence system (Thermo Fisher Scientific/Thermo pierce) was used.
2.4. Transwell migration and invasion assay
To assess cell migration in vitro, shPTBP1 and NC lentivirus‐infected MDA‐MB‐231 cells (1 × 104cells in 100 μl DMEM) were separately placed in the top chamber of transwell chambers (8‐μm BioCoat Control Inserts, Corning Costar). The lower chamber was filled with 600 μl DMEM supplemented with 10% FBS. After 24 hr incubation at 37°C, the cells were fixed by 85% alcohol and stained with 0.4% trypan blue. The cells in the top chambers were removed with cotton swabs very carefully and counted (five random fields per well at 100× magnification) under a light microscope. For invasion assay, 5 × 104 cells were plated in the matrigel‐coated chamber and the migration assay was performed.
2.5. MTT proliferation assay
shPTBP1 and nonspecific control (NC) stable transfected MDA‐MB‐231 cells were seeded in 96‐well plates (3 × 103) for cell viability assay. The cells were incubated with 5 μl 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) (10 mg/ml) at 37°C for 4 hr and then the supernatants were discarded carefully. 200 μl dimethyl sulfoxide (DMSO) was added to dissolve the formazan product for 20 min at room temperature. The absorbance of each sample was measured at 490 nm.
2.6. Lentiviral infection
PTBP1 short hairpin RNAs (PTBP1‐shRNA) and control lentivirus were obtained from Shanghai Genechem Co., Ltd. The PTBP1‐shRNA 1# sequence was 5′‐TCGTCAAAGGATTCAAGTT‐3′; the PTBP1‐shRNA 2# sequence was 5′‐CAACGTCAAGTACAACAAT‐3′; the PTBP1‐shRNA 3# sequence was 5′‐AGCCCATCTACATCCAGTT‐3′ and the shRNA control sequence was 5′‐ TTCTCCGAACGTGTCACGT‐3′. MDA‐MB‐231 cells were seeded into 12‐well plates overnight. Then, the cells were infected with PTBP1‐shRNA 1#, PTBP1‐shRNA 2#, PTBP1‐shRNA 3# and control lentivirus following the manufacturer's guidelines (GeneChem, China); 5 μg/ml puromycin (Sigma) was added to the medium to select infected cells. 5 days after infection, fluorescence microscopy was used to detect the lentiviral infection rate.
2.7. RNA isolation and quantitative Real time‐Polymerase chain reaction
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad) from cells and tissues. Total RNA (1 μg) was used for the synthesis of complementary deoxyribonuceic acid (cDNA) using the PrimeScript™ RT Reagent Kit (Takara, China). The sequences of the primers were as follows (5′–3′): PTBP1(CTGAGGATCCATGTCTGGTTATTCTAGTG and TTACTCTCGAGTTACTGGGAATATCCGGTT); GAPDH (ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA). Quantitative real time‐PCR was performed using the SYBR green mix (Applied Biosystems/Takara). The reactions were performed with a 7,500 Fast Real‐Time PCR System (Applied Biosystems) as follows: 1 cycle of 95 °C for 1 min, 50 cycles of 95°C for 10 s, 55°C for 30 s, 1 cycle of 95°C for 1 min, 55°C for 30 s, 95°C for 30 s.
2.8. Clone formation
PTBP1‐shRNA and control lentiviral infection cells were seeded in matrigel (BD) precoated six‐well plates. After 14 days, the cells were photographed and counted by an inverted microscope.
2.9. Immunohistochemical staining
Paraffin sections cut from paraffin‐embedded breast cancer tissues were dewaxed and rehydrated. Immunohistochemical staining assay was used to detected the expression of PTBP1 in all tumor sections with the PTBP1 antibody (1:250; Abcam) and ElivisionTMplus (Maixin Biotechnology Co., Ltd, China). Then the nucleus was stained with hematoxylin (Maixin Biotechnology Co., Ltd). After hydration and transparent, the sections were sealed with neutral resins and photographed.
2.10. Tumor‐bearing nude mice
shPTBP1 and control MDA‐MB‐231 cells were trypsinized, washed once with phosphate buffer solution (PBS), and resuspended in PBS (2 × 107/ml). One hundred micro liters of cell solution was injected into the bilateral axillary of BALB/c nude mice (n = 5 per group). The tumor size of each mouse was measured every 3 days, and 36 days after injection, the mice were anesthetized and then euthanized. The tumors were extracted and fixed in 4% paraformaldehyde after washing in PBS.
2.11. Statistical analysis
Differences between the two groups were estimated with one‐way analysis of variance and the t test. All the data were analyzed by Statistical SPSS Version 17.0. A P value of < 0.05 was considered statistically significant.
3. RESULTS
3.1. PTBP1 expression is upregulated in breast cancer cell lines and clinical tumor samples
Using western blot, we examined the expression of PTBP1 in a series of the breast cancer cell lines, MCF‐7, ZR‐75‐1, ZR‐75‐30, MDA‐MB‐453, HCC1937, Hs578T, MDA‐MB231, and normal breast epithelia MCF‐10A. The expression of PTBP1 is upregulated in breast cancer cell lines compared with normal epithelia (1A). We then examined the expression of PTBP1 in breast cancers and the adjacent normal samples in the same patients. As a result, the expression levels of PTBP1 in the clinical breast cancer samples examined by western blot were extremely upregulated compared with those in the normal tissues (Figure 1b). The result was further confirmed by immunohistochemical staining (Figure 1c). We further analyzed the relationship between PTBP1 and clinicopathological parameters. The analyzed parameters were tumor size, axillary lymph node metastasis (pN), ER, PR, pathological stage, and her‐2. We found that PTBP1 was correlated with her‐2 expression, lymph node metastasis, and pathological stage (Table 1). These results indicate that PTBP1 is associated with and may be involved in the neoplastic transformation of breasts; its upregulation is likely an early event in the transformation process.
Figure 1.
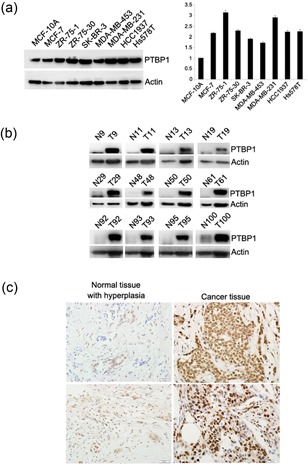
PTBP1 expression is upregulated in breast cancer cell lines and clinical tumor samples. (a) Overexpression of PTBP1 in various breast cancer cell lines. The expression of PTBP1 in a series of the breast cancer cell lines MCF‐7, ZR‐75‐1, ZR‐75‐30, MDA‐MB‐453, HCC1937, Hs578T, MDA‐MB231, and normal breast epithelia MCF‐10A determined by western blot analysis. Actin was used as the control. (b) The expression of PTBP1 in 137 pairs of breast cancer tissue compared with normal tissue as determined by western blot analysis. Actin was used as the control. (c) Immunohistochemical staining for PTBP1 in human breast cancers and normal tissue. PTBP1 mainly localized in the nucleus of tumor cells. The expression of PTBP1 was upregulated in breast cancer tissue compared with the normal tissue. PTBP1, polypyrimidine tract binding protein 1 [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
PTBP1 expression during breast cancer
| PTBP1 expression | ||||
|---|---|---|---|---|
| Feature | Low | High | ||
| Tumor size (cm) | ||||
| <5 | 101 | 38 | 63 | 0.672 |
| ≥5 | 36 | 15 | 21 | |
| Lymph node metastasis (pN) | ||||
| No | 69 | 35 | 34 | 0.003** |
| Yes | 68 | 18 | 50 | |
| ER | ||||
| Positive | 95 | 32 | 63 | 0.072 |
| Negative | 42 | 21 | 21 | |
| PR | ||||
| Positive | 86 | 31 | 55 | 0.414 |
| Negative | 51 | 22 | 29 | |
| Her2 | ||||
| Positive | 57 | 13 | 44 | 0.001** |
| Negative | 80 | 40 | 40 | |
| Pathological stage (pStage) | ||||
| Stages I, II | 46 | 26 | 20 | 0.002** |
| Stages III, IV | 91 | 27 | 64 | |
Note. ER, estrogen receptor; PR, progesterone receptor; PTBP1, polypyrimidine tract binding protein 1
Indicated statistical significance (P < 0.01)
3.2. Knockdown of PTBP1 expression inhibits breast cancer cell proliferation, migration, and invasion
The frequent overexpression of PTBP1 in breast cancer tissues and breast cancer cell lines but not in normal tissues and epithelia implicated that PTBP1 may play an important role in breast cancer proliferation, migration, and invasion. To address the question whether the overexpressed PTBP1 has any functional role in breast cancer cells, the MDA‐MB‐231 cell line was selected and three shRNAs, which specifically target PTBP1, and one control shRNA were synthesized. We established sublines of breast cancer cell lines MDA‐MB‐231 to express virus‐induced PTBP1 shRNAs. Cells were infected with PTBP1‐shRNA and control lentivirus. Five days after infection, fluorescence microscopy was used to detect the lentiviral infection rate and the infection rate was over 80% in each group (Figure 2a). We tested three effective PTBP1 shRNAs that target different regions of the PTBP1 mRNA, shPTBP1 1#, shPTBP1 2#, and shPTBP1 3#. After infection, the knockdown of PTBP1 was confirmed in mRNA levels (Figure 2b). The result was also confirmed in protein levels (Figure 2c). We then selected shPTBP1 2# and shPTBP1 3# for further investigation. We compared the growth of shPTBP1 2#, shPTBP1 3#, and the control group. We found that the cell growth was significant suppressed in the shPTBP1 2# and shPTBP1 3# groups compared with the control group (Figure 3a). A significant reduction in colony numbers was observed infected with PTBP1 shRNA compared the control group (Figure 3b). One hallmark of cancer cells is their invasive properties. To determine whether PTBP1 overexpression contributes to this malignant phenotype, we examined whether PTBP1 knockdown interfered with the in vitro invasiveness of MDA‐MB‐231, a highly invasive breast cancer cell line. To investigate the role of PTBP1 in cell migration and invasion, we conducted transwell migration/invasion assays. The results indicated that PTBP1 knockdown remarkably reduced the migratory and invasive ability of breast cancer cells (Figure 3c). These results indicate that knockdown of PTBP1 indeed inhibits the invasive behavior of these breast cancer cells, a finding that would appear to have therapeutic consequences for breast cancer cells.
Figure 2.
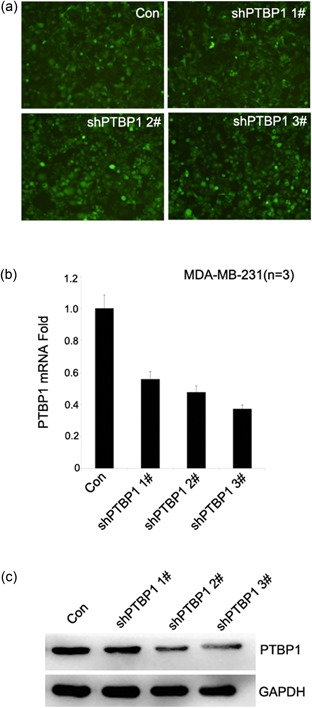
The synthesization of PTBP1‐knockdown cell lines. (a) Fluorescence was used to detect the lentiviral infection rate. The lentivirus infection rate is high in shPTBP1 1#, shPTBP1 2#, and shPTBP1 3# sublines. The lentiviral infection rate and the infection rate were over 80% in each group. (b) RT‐PCR demonstrating significant shRNA‐mediated knockdown of PTBP1 expression in shPTBP1 1#, shPTBP1 2#, and shPTBP1 3# sublines but not in control subline. (c) PTBP1 expression is substantially suppressed in shPTBP1 1#, shPTBP1 2#, and shPTBP1 3# sublines but not in control subline using western blot. GADPH was used as a loading control. GADPH, glyceraldehyde‐3‐phosphate dehydrogenase; PTBP1, polypyrimidine tract binding protein 1; RT‐PCR, real time‐polymerase chain reaction; shRNA, short hairpin RNA [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
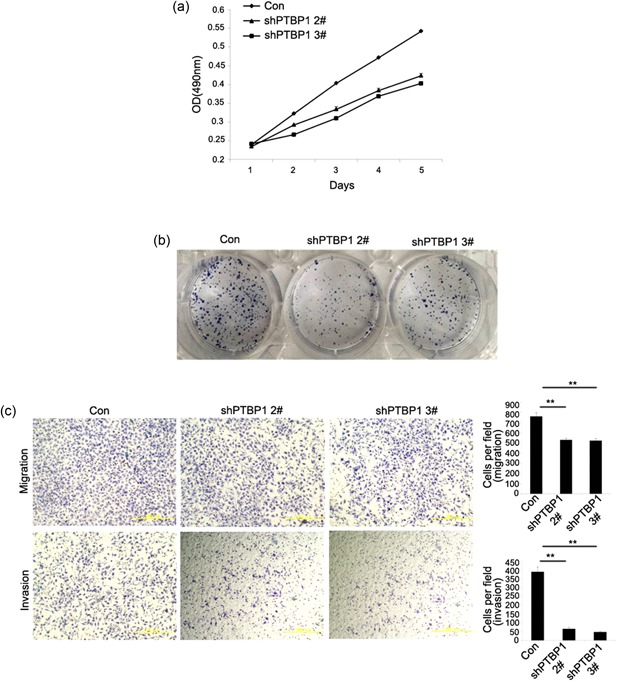
Knockdown of PTBP1 expression inhibits in vitro proliferation and invasiveness of breast cancer cells. (a) MTT assay evaluation of influence of PTBP1 knockdown on cell viability. Cell growth is inhibited in shPTBP1 1# and shPTBP1 3# compared with control subline. (b) Colony formation assays were performed to test the colony formation ability in respective cell lines after infection with shRNA and the controls. (c) Cell migration and invasion assays were determined after infection. Photos were taken under an inverse microscope 10×. Data are presented as mean ± standard deviation and are representative of three repeated experiments. **P < 0.05. PTBP1, polypyrimidine tract binding protein 1; MTT, 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide; shRNA, short hairpin RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Knockdown of PTBP1 expression inhibits breast cancer cell proliferation in vivo
To show the effect of decreased PTBP1 expression levels in breast cancer cells in vivo, we generated xenografts in nude mice. MDA‐MB‐231 cell infected with lentivirus expressing PTBP1 shRNA or control shRNA were subcutaneously injected into five nude mice in total. The first day of lentiviral shRNA injection was recorded as day 0 and after 3 weeks the tumor size was measured every 3 days. At the 36th day, these mice were killed and tumor weight and volume were measured. The tumors derived from the PTBP1 shRNA group were significantly smaller in size and much lighter in weight than the control, when the tumors were isolated (Figure 4a–d). These findings indicated that PTBP1 could even affect the proliferation of breast cancer cells in vivo.
Figure 4.
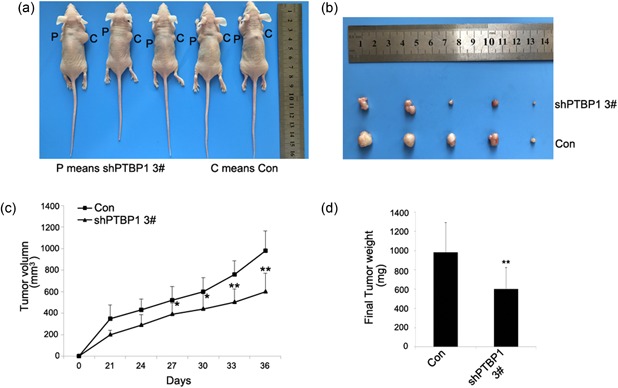
Tumor bearing nude mice. (a) Subcutaneous tumors in nude mice 36 days after adenovirus injection. (b) Subcutaneous tumors isolated from nude mice 36 days after adenovirus injection. (c) Growth curves of tumors in nude mice. Growth curves represents days after adenovirus injection in x axis and tumor volume in y axis. Data form are presented as mean ± SD. *P < 0.05 and **P < 0.01 compared with control group and shRNA group. (d) Tumors were isolated and weights were measured on the 36th day of the two groups above. Data form are presented as mean ± standard deviation. **P < 0.01. shRNA, short hairpin RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.4. PTBP1 contributed to the proliferation of breast cancer through the activation of PTEN/Akt signal pathway and knockdown of PTBP1 induce autophagy
The PTEN/Akt signal pathway was a classic growth signaling pathway that activated in various cancers. To determine whether there was a change in the expression of PTEN and protein kinase B (Akt) in PTBP1‐knockdown cells, we examined PTBP1‐knockdown sublines by western blot. We found that the expression of p‐Akt decreased in PTBP1‐knockdown cells compared with controls (Figure 5a). With the overexpression of PTBP1, the expression of p‐Akt increased compared with controls (Figure 5b). The expression of PTEN increased in PTBP1‐knockdown cells compared with the controls (Figure 5c), and with the overexpression of PTBP1, the expression of PTEN decreased compared with the controls (Figure 5d). These data suggested that PTBP1 knockdown induced cell growth inhibition could be partially mediated by an altered PTEN/Akt signal pathway. Knockdown of PTBP1 was reported to lead to autophagy in colorectal cancer. In our study, we found that knockdown of PTBP1 induced the transition of the LC3BI to LC3BII in cancer cells (Figure 5e) and overexpression of PTBP1 reduced the transition of the LC3BI to LC3BII (Figure 5f), which was a significant event in autophagy. To investigate whether PTBP1 can affect the PTEN/Akt signal pathway in breast cancer samples, we examined paired samples from 137 breast cancer patients by western blot analysis. The levels of PTBP1 and p‐Akt were high (Figure 6a). The relationship between PTBP1 and p‐Akt was significant (Figure 6b).
Figure 5.
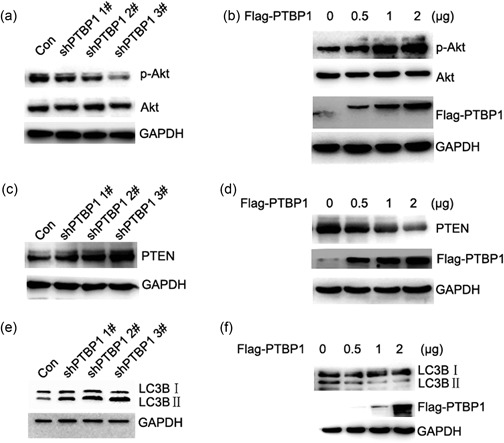
Knockdown of PTBP1 leads to upregulation of PTEN and decreased expression of p‐Akt and cell growth. (a) To validate the effct of PTBP1 on P‐Akt western blot analysis was performed. Expression of p‐Akt decreased in PTBP1‐knockdown cells compared with control. GADPH was used as a loading control. (b) With the overexpression of PTBP1, the expression of p‐Akt increased compared with control using western blot assay. GADPH was used as a loading control. (c) To validate the effct of PTBP1 on PTEN, western blot analysis was performed. The expression of PTEN increased in PTBP1‐knockdown cells compared with the control. GADPH was used as a loading control. (d) The overexpression of PTBP1 decreased the expression of PTEN compared with the controls using western blot assay. GADPH was used as a loading control. (e) To validate the effct of PTBP1 on autophagy, the expression level of LC3BI and LC3BII was examined by western blot analysis. Knockdown of PTBP1 induced the transition of the LC3BI to LC3BII. GADPH was used as a loading control. (f) Overexpression of PTBP1 reduced the transition of the LC3BI to LC3BII using western blot analysis. GADPH was used as a loading control. GADPH, glyceraldehyde‐3‐phosphate dehydrogenase; p‐AkPTBP1, polypyrimidine tract binding protein 1; PTEN, phosphatase and tensin homolog
Figure 6.
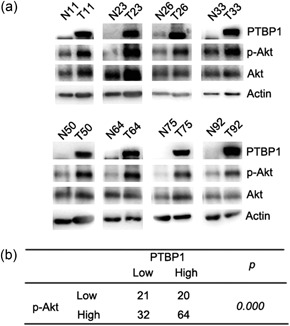
Increased expression levels of PTBP1 and p‐Akt were demonstrated in clinical tumor samples from breast cancer patients. (a) PTBP1, p‐Akt, and Akt expressions were analyzed in 137 breast cancer samples as determined by western blot. Actin was used as the control. (b) The relationship between PTBP1 and p‐Akt was significant. P < 0.01. PTBP1, polypyrimidine tract binding protein 1; p‐Akt, p‐protein kinase B
4. DISCUSSION
Breast cancer is one of the most common malignancies in middle aged and elderly women globally, representing 1.20 million cases annually in women worldwide (Chen et al., 2016). In recent years, significant progress has been made in the treatment of breast cancer as more basic and clinical studies were conducted, and the study on breast cancer treatment has become one of the hot topics of cancer research (Cote et al., 1999). Invasion and migration is the hallmark of malignant tumors as well as the major cause for breast cancer death (Auweter & Allain, 2008; He et al., 2004). However, the development details and underlying pathological mechanisms remain to be determined. The PTB, also known as PTBP1 or polypyrimidine tract binding protein 1 (hnRNPI), is one of the most investigated RBP in vertebrates involved in almost all steps of mRNA regulation during tumorigenesis, due to its RNA‐binding activity. PTBP1 is generally described as a widely expressed factor in adult tissues, and accordingly it is present in most of the cell lines studied (Wang et al., 2008). However, its amount can strongly vary from tissue to tissue or depending on the cellular differentiation states. The expression levels of PTBP1 have been found to be elevated in brain tumors (He et al., 2004; He et al., 2007), ovarian tumors (Jin et al., 2000), and different malignant cell lines (McCutcheon et al., 2004). Furthermore, high expression of PTBP1 has been demonstrated to be associated with the aggressive behavior of several types of cancer, especially in glioma and ovarian tumors (He et al., 2007; Jin et al., 2000). Thus, PTBP1 plays an important role and has different functions in tumorigenesis by regulating the amounts of target genes associated with malignancy.
Our data revealed that PTBP1 was significantly higher in breast cancer tissues and breast cancer cell lines compared with normal breast tissue and normal human breast epithelia, as demonstrated by the immunohistochemistry and western blot analysis (Figure 1), consistent with previous studies (He et al., 2014; He, Yuan, & Yang, 2015). A recent study found that PTBP1 is overexpressed in ovarian tumors and colorectal cancer (He et al., 2007; Takahashi et al., 2015) indicating that PTBP1 is closely associated with the pathogenesis and development of cancer. However, the correlation of PTBP1 with clinical characteristics has not been clarified. Further analysis was performed to investigate the relationship between PTBP1 expression in breast cancer tissues and clinicopathological characteristics of cancer. The results revealed that the level of PTBP1 expression in breast cancer tissue was positively correlated with her‐2, lymph node metastasis, and pathological stage (Table 1).
The mechanism underlying the tumor promoting function of PTBP1 in breast cancer was further studied by reducing PTBP1 expression in the human breast cancer cell lines. As reported previously, PTBP1 has been involved in several biological functions crucial for cancer development, including proliferation (Cheung et al., 2009; He et al., 2007; He et al., 2014; Sugiyama et al., 2016), migration and invasion (Cheung et al., 2009; He et al., 2007; He et al., 2014). In our study, we discovered that knockdown of PTBP1 significantly inhibited breast cancer cell proliferation in vitro. Similarly, recent study has found that PTBP1 downregulation suppressed cell proliferation in colorectal cancer in vitro (Takahashi et al., 2015). Meanwhile, we found that PTBP1 knockdown attenuated the migration and invasion of breast cancer cells, whereas overexpression of it enhanced these malignant properties. Consistent with our findings, PTBP1 knockdown inhibited the migration of glioma cells (Cheung et al., 2009), the migration and invasion of ovarian tumor cells (He et al., 2007; He et al., 2015). Combined with clinical association of lymph node metastasis, these data indicated that PTBP1 prompted metastasis in breast cancer and might be an effective antimetastasis target for therapy. The results demonstrate that the proliferation and migration of breast cancer cells were reduced with the suppression of PTBP1, indicating that PTBP1 may affect the malignancy of breast cancer cells. The function of the significance upregulation of PTBP1 has been studied in vitro and in vivo. By shRNA mediated knockdown of PTBP1, we focused on the knockdown effect in MDA‐MB‐231 breast cancer cell lines. PTBP1 knockdown inhibited short‐term breast cancer cell proliferation in MTT assays, long‐term colony formation and by the subcutaneous tumor model in nude mice and reduced the invasive capacity of migration and invasion by Transwell Migration/invasion assays, consistent with other results in tumor cells from multiple origins (Takahashi et al., 2015; Wang et al., 2008). The final biological effect of PTBP1 depended on the combination of its different target transcripts, transcription levels of mRNAs, post‐transcriptional regulation, and the network of genes (Cote, Dupuis, & Wu, 2001; Lin, Wang, & Tseng, 2013; Ohno, Shibayama, Sato, Tokunaga, & Yoshida, 2011; Sauliere et al., 2006; Xue et al., 2009).
The PTEN/Akt signal pathway has been the most studied pathway in tumorigenesis in recent years. It can regulate cell cycle, cell apoptosis and participates in cell proliferation, migration, and invasion. Mechanistically, the p‐Akt/Akt ratio is remarkably decreased when PTBP1 is silenced and significantly increased when PTBP1 is overexpressed by western blot. Furthermore, we detected the expression level of PTEN and found that it was downregulated by PTBP1. Consistent with our findings, PTBP1 is reported to mediate the PTEN/Akt signal pathway in bladder cancer (Takai et al., 2017).
Autophagy is a highly conserved physiological phenomenon in most eukaryotic cells and a cellular material recycling mechanism that is dependent on the lysosomes. Autophagy is closely related to the development of malignant tumors. In a variety of adverse circumstances, autophagy protects survival of tumor cells as an emergency mechanism, whereas initiation of autophagic cell death clears the tumor cells. Knockdown of PTBP1 induces the transition of the LC3BI to LC3BII in cancer cells, which can prompt autophagy. Mechanistically, the LC3BII/LC3BI ratio was remarkably increased when PTBP1 silenced and significantly decreased when PTBP1 overexpressed using western blot analysis. Consistent with our findings, PTBP1 is reported to mediate the LC3B isoform switching from LC3BII to LC3BI in bladder cancer (Takai et al., 2017). These results strongly suggested that PTBP1 positively regulated the proliferation of breast cancer cells through the combination regulation of PTEN/Akt signal pathway and the transition of the LC3BI to LC3BII in autophagy.
In conclusion, it is our novel discovery that PTBP1 is upregulated in breast cancer and associates with her‐2, lymph node metastasis and pathological stage. Moreover, PTBP1 promotes breast cancer cell proliferation, migration, and invasion by the combination of regulation of the PTEN/Akt signal pathway and autophagy. Therefore, PTBP1 is a potential biomarker and molecular therapeutic target for breast cancer.
In summary, a high expression of PTBP1 is important in the development of breast cancer, possibly by influencing the proliferation and migration of cancer cells. Detection and interference of PTBP1 have a certain implication for guiding the treatment and prognosis of breast cancer in clinical practice.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
ACKNOWLEDGMENT
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81602564 and 81773163)
Wang X, Li Y, Fan Y, Yu X, Mao X, Jin F. PTBP1 promotes the growth of breast cancer cells through the PTEN/Akt pathway and autophagy. J Cell Physiol. 2018;233:8930–8939. 10.1002/jcp.26823
References
REFERENCES
- Auweter, S. D. , & Allain, F. H. (2008). Structure‐function relationships of the polypyrimidine tract binding protein. Cellular and Molecular Life Science, 65(4), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Zheng, R. , Baade, P. D. , Zhang, S. , Zeng, H. , Bray, F. , … He, J. (2016). Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians, 66(2), 115–132. [DOI] [PubMed] [Google Scholar]
- Cheung, H. C. , Hai, T. , Zhu, W. , Baggerly, K. A. , Tsavachidis, S. , Krahe, R. , & Cote, G. J. (2009). Splicing factors PTBP1 and PTBP2 promote proliferation and migration of glioma cell lines. Brain, 132(Pt 8), 2277–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, C. A. , Gautreau, D. , Denegre, J. M. , Kress, T. L. , Terry, N. A. , & Mowry, K. L. (1999). A Xenopus protein related to hnRNP I has a role in cytoplasmic RNA localization. Molecular Cell, 4(3), 431–437. [DOI] [PubMed] [Google Scholar]
- Cote, J. , Dupuis, S. , & Wu, J. Y. (2001). Polypyrimidine track‐binding protein binding downstream of caspase‐2 alternative exon 9 represses its inclusion. Journal of Biological Chemistry, 276(11), 8535–8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Arslan, A. D. , Ho, T. T. , Yuan, C. , Stampfer, M. R. , & Beck, W. T. (2014). Involvement of polypyrimidine tract‐binding protein (PTBP1) in maintaining breast cancer cell growth and malignant properties. Oncogenesis, 3, e84–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Ee, P. L. , Coon, J. S. , & Beck, W. T. (2004). Alternative splicing of the multidrug resistance protein 1/ATP binding cassette transporter subfamily gene in ovarian cancer creates functional splice variants and is associated with increased expression of the splicing factors PTB and SRp20. Clinical Cancer Research, 10(14), 4652–4660. [DOI] [PubMed] [Google Scholar]
- He, X. , Pool, M. , Darcy, K. M. , Lim, S. B. , Auersperg, N. , Coon, J. S. , & Beck, W. T. (2007). Knockdown of polypyrimidine tract‐binding protein suppresses ovarian tumor cell growth and invasiveness in vitro. Oncogene, 26(34), 4961–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Yuan, C. , & Yang, J. (2015). Regulation and functional significance of CDC42 alternative splicing in ovarian cancer. Oncotarget, 6(30), 29651–29663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, W. , McCutcheon, I. E. , Fuller, G. N. , Huang, E. S. , & Cote, G. J. (2000). Fibroblast growth factor receptor‐1 alpha‐exon exclusion and polypyrimidine tract‐binding protein in glioblastoma multiforme tumors. Cancer Research, 60(5), 1221–1224. [PubMed] [Google Scholar]
- Lin, S. , Wang, M. J. , & Tseng, K. Y. (2013). Polypyrimidine tract‐binding protein induces p19(Ink4d) expression and inhibits the proliferation of H1299 cells. PLoS One, 8(3), e58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorian, M. , Schwartz, S. , Clark, T. A. , Hollander, D. , Tan, L. Y. , Spellman, R. , … Smith, C. W. (2010). Position‐dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nature Structural & Molecular Biology, 17(9), 1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon, I. E. , Hentschel, S. J. , Fuller, G. N. , Jin, W. , & Cote, G. J. (2004). Expression of the splicing regulator polypyrimidine tract‐binding protein in normal and neoplastic brain. Neuro Oncology, 6(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, S. , Shibayama, M. , Sato, M. , Tokunaga, A. , & Yoshida, N. (2011). Polypyrimidine tract‐binding protein regulates the cell cycle through IRES‐dependent translation of CDK11(p58) in mouse embryonic stem cells. Cell Cycle, 10(21), 3706–3713. [DOI] [PubMed] [Google Scholar]
- Pesson, M. , Volant, A. , Uguen, A. , Trillet, K. , De La Grange, P. , Aubry, M. , … Corcos, L. (2014). A gene expression and pre‐mRNA splicing signature that marks the adenoma‐adenocarcinoma progression in colorectal cancer. PLoS One, 9(2), e87761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauliere, J. , Sureau, A. , Expert‐Bezancon, A. , & Marie, J. (2006). The polypyrimidine tract binding protein (PTB) represses splicing of exon 6B from the beta‐tropomyosin pre‐mRNA by directly interfering with the binding of the U2AF65 subunit. Molecular and Cellular Biology, 26(23), 8755–8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, T. , Taniguchi, K. , Matsuhashi, N. , Tajirika, T. , Futamura, M. , Takai, T. , … Yoshida, K. (2016). MiR‐133b inhibits growth of human gastric cancer cells by silencing pyruvate kinase muscle‐splicer polypyrimidine tract‐binding protein 1. Cancer Prevention Research, 107(12), 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Nishimura, J. , Kagawa, Y. , Kano, Y. , Takahashi, Y. , Wu, X. , … Yamamoto, H. (2015). Significance of polypyrimidine tract‐binding protein 1 expression in colorectal cancer. Molecular Cancer Therapeutics, 14(7), 1705–1716. [DOI] [PubMed] [Google Scholar]
- Takai, T. , Yoshikawa, Y. , Inamoto, T. , Minami, K. , Taniguchi, K. , Sugito, N. , … Azuma, H. (2017). A novel combination RNAi toward Warburg effect by replacement with miR‐145 and silencing of PTBP1 induces apoptotic cell death in bladder cancer cells. International Journal of Molecular Sciences, 18(1), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen, K. , Sorensen, K. D. , Brems‐Eskildsen, A. S. , Modin, C. , Gaustadnes, M. , Hein, A. M. , … Orntoft, T. F. (2008). Alternative splicing in colon, bladder, and prostate cancer identified by exon array analysis. Molecular & Cellular Proteomics, 7(7), 1214–1224. [DOI] [PubMed] [Google Scholar]
- Venables, J. P. (2006). Unbalanced alternative splicing and its significance in cancer. Bioessays, 28(4), 378–386. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Norton, J. T. , Ghosh, S. , Kim, J. , Fushimi, K. , Wu, J. Y. , … Huang, S. (2008). Polypyrimidine tract‐binding protein (PTB) differentially affects malignancy in a cell line‐dependent manner. Journal of Biological Chemistry, 283(29), 20277–20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollerton, M. C. , Gooding, C. , Wagner, E. J. , Garcia‐Blanco, M. A. , & Smith, C. W. (2004). Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense‐mediated decay. Molecular Cell, 13(1), 91–100. [DOI] [PubMed] [Google Scholar]
- Xue, Y. , Zhou, Y. , Wu, T. , Zhu, T. , Ji, X. , Kwon, Y. S. , … Zhang, Y. (2009). Genome‐wide analysis of PTB‐RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Molecular Cell, 36(6), 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , & Manley, J. L. (2013). Misregulation of pre‐mRNA alternative splicing in cancer. Cancer Discovery, 3(11), 1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]


