Abstract
Aim
To evaluate the potential for semaglutide to help people with type 2 diabetes (T2D) achieve glycated haemoglobin (HbA1c) targets while avoiding unwanted outcomes, such as weight gain, hypoglycaemia and gastrointestinal (GI) side effects.
Materials and methods
Data from the phase IIIa SUSTAIN 1 to 5 clinical trials were analysed. Participants had inadequately controlled T2D and were drug‐naïve (SUSTAIN 1) or on a range of background treatments (SUSTAIN 2 to 5). The main protocol‐specified composite endpoint was the proportion of participants achieving HbA1c <53 mmol/mol (7.0%) at end of treatment (30 or 56 weeks) without weight gain and with no severe or blood glucose (BG)‐confirmed symptomatic hypoglycaemia. A post hoc composite endpoint was the proportion of participants achieving the primary composite endpoint without moderate or severe GI adverse events (AEs).
Results
Across the SUSTAIN trials 1 to 5, 3918 participants with T2D were randomized to once‐weekly subcutaneous semaglutide 0.5 mg, 1.0 mg, or comparators (placebo, sitagliptin 100 mg, exenatide extended release 2.0 mg or insulin glargine). The proportion of participants achieving HbA1c <53 mmol/mol (7.0%) with no weight gain and no severe/BG‐confirmed symptomatic hypoglycaemia was 47% to 66% (semaglutide 0.5 mg) and 57% to 74% (semaglutide 1.0 mg) vs 7% to 19% (placebo) and 16% to 29% (active comparators; all P < .0001). More participants achieved the primary composite endpoint with no moderate or severe GI AEs with semaglutide vs comparators (all P < .0001).
Conclusion
Semaglutide helped more people with T2D achieve HbA1c targets than did comparators in the SUSTAIN 1 to 5 trials, while avoiding unwanted outcomes such as weight gain, hypoglycaemia and GI side effects.
Keywords: GLP‐1, glycaemic control, hypoglycaemia, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is a chronic disease characterized by insulin resistance and a progressive decline in β‐cell function.1 Initial management of T2D involves lifestyle changes and monotherapy with metformin.1, 2 Often, however, as glycaemic control deteriorates, combination therapy with other oral antidiabetic drugs (OADs) is required, with or without injectable therapies such as insulin and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs).1, 2
The optimum treatment for T2D should involve patient‐oriented treatment goals that extend beyond glycated haemoglobin (HbA1c) reduction alone, such as minimizing the risk of unwanted effects, including hypoglycaemia and weight gain.3, 4
Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone that regulates food intake and improves glycaemia in a blood glucose (BG)‐dependent manner.5 GLP‐1RAs are effective at reducing HbA1c, body weight and inducing satiety.1, 6 Unlike therapy with insulin and sulphonylureas, GLP‐1RAs act by inducing glucose‐dependent insulin secretion and are associated with a low risk of hypoglycaemia and weight gain.1, 6, 7, 8 GLP‐1RAs are generally well tolerated,1 although gastrointestinal (GI) adverse events (AEs) are commonly observed across the class.2, 9
Semaglutide (Novo Nordisk, Bagsværd, Denmark) is a novel GLP‐1 analogue recently approved by the US Food and Drug Administration for the treatment of T2D. Its amino acid sequence is 94% homologous to that of native GLP‐1,10 and it has a half‐life of ~1 week, which makes it appropriate for once‐weekly subcutaneous (s.c.) administration.10, 11
SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) is a global phase III clinical trial programme, designed to evaluate the efficacy and safety of once‐weekly s.c. semaglutide across a range of people with T2D, from those who are drug‐naïve to those receiving OADs and/or insulin.12, 13, 14, 15, 16, 17
Across the SUSTAIN trials, significantly more semaglutide‐treated participants achieved HbA1c targets versus comparators, as well as clinically relevant reductions in body weight and a generally low risk of hypoglycaemia.12, 13, 14, 15, 16, 17 In the SUSTAIN 6 trial, semaglutide also showed a 26% cardiovascular risk reduction vs placebo in participants with T2D, as measured by the composite endpoint of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke.17 The most common AEs experienced with semaglutide across the SUSTAIN programme were GI in nature.12, 13, 14, 15, 16, 17 The SUSTAIN 7 trial evaluated once‐weekly semaglutide vs once‐weekly dulaglutide in people with T2D18; however, data from SUSTAIN 7 were not available at the time of this analysis.
In the present study, data from the SUSTAIN 1 to 5 trials12, 13, 14, 15, 16 were analysed to evaluate the proportion of people with T2D achieving clinically relevant HbA1c targets with semaglutide while avoiding unwanted effects such as weight gain, hypoglycaemia and GI side effects.
2. METHODS
2.1. Trial design
The designs of the SUSTAIN 1 to 5 trials (NCT02054897, NCT01930188, NCT01885208, NCT02128932 and NCT02305381) have previously been reported in full12, 13, 14, 15, 16 and are summarized in Table 1. Briefly, adults with T2D were randomized to treatment with semaglutide or comparators for 30 or 56 weeks (Table 1). Comparators included placebo, sitagliptin, exenatide extended release (ER) and insulin glargine (IGlar).
Table 1.
SUSTAIN 1 to 5 trial designs
| SUSTAIN 1 | SUSTAIN 2 | SUSTAIN 3 | SUSTAIN 4 | SUSTAIN 5 |
|---|---|---|---|---|
| Trial design | ||||
| Double‐blind, randomized, multicentre | Double‐blind, randomized, multicentre | Open‐label, randomized, multicentre | Open‐label, randomized, multicentre | Double‐blind, randomized, multicentre |
| Inclusion criteria/background medication | ||||
| Male or female participants diagnosed with T2D, aged ≥18 yearsa | ||||
| Drug‐naïve; treated with diet/exercise only | On stable treatmentb with MET, TZD or MET + TZD | On stable treatmentb with 1–2 OADs (MET, TZD, MET+TZD, SU) | Insulin‐naïve and on stable treatmentb with MET or MET+SU | On stable treatmentb with basal insulin alone or basal insulin+MET |
| HbA1c 7.0–10.0% | HbA1c 7.0–10.5% | HbA1c 7.0–10.5% | HbA1c 7.0–10.0% | HbA1c 7.0–10.0% |
| Exclusion criteria | ||||
| Treatment with glucose‐lowering agent 90 days prior to screeningc | Treatment with glucose‐lowering agent, other than those stated in the inclusion criteria, 90 days prior to screeningc | Treatment with glucose‐lowering agent, other than those stated in the inclusion criteria, 90 days prior to screeningc | Treatment with glucose‐lowering agent, other than those stated in the inclusion criteria, 90 days prior to screeningc | Treatment with any glucose‐lowering agent other than stated in the inclusion criteria, 90 days prior to screeningc |
| eGFR <30 mL/min/1.73 m2 d | eGFR <60 mL/min/1.73 m2 d | eGFR <60 mL/min/1.73 m2 d | eGFR <30 mL/min/1.73 m2 d | eGFR <30 mL/min/1.73 m2 d |
| >3 episodes of severe hypoglycaemia within 6 months prior to screening | >3 episodes of severe hypoglycaemia within 6 months prior to screening | |||
| History of pancreatitis, screening calcitonin ≥50 ng/L, personal or family history of MTC or MEN2, acute coronary or cerebrovascular event within 90 days before randomisation, heart failure (NYHA class IV), known proliferative retinopathy or maculopathy requiring acute treatment | ||||
| Treatment regimen | ||||
| Treatments given s.c. once‐weekly: Semaglutide 0.5 or 1.0 mg Or Placebo 0.5 or 1.0 mg |
Semaglutide 0.5 or 1.0 mg s.c. Once‐weekly + placebo oral once daily Or Sitagliptin oral once‐daily + placebo 0.5 mg or 1.0 mg s.c. Once‐weekly |
Treatments given s.c. once‐weekly: Semaglutide 1.0 mg Or Exenatide ER 2.0 mg |
Semaglutide 0.5 or 1.0 mg s.c. Once‐weekly Or IGlar starting from 10 IU, once‐daily |
Treatments given s.c. once‐weekly: Semaglutide 0.5 or 1.0 mg Or Placebo 0.5 or 1.0 mg |
| Treatment duration | ||||
| 30 weeks | 56 weeks | 56 weeks | 30 weeks | 30 weeks |
| Primary endpoints | ||||
| Change in HbA1c from baseline to week 30 | Change in HbA1c from baseline to week 56 | Change in HbA1c from baseline to week 56 | Change in HbA1c from baseline to week 30 | Change in HbA1c from baseline to week 30 |
Abbreviations: eGFR, estimated glomerular filtration rate; ER, extended release; HbA1c, glycated haemoglobin; IGlar, insulin glargine; MEN2, multiple endocrine neoplasia type 2; MET, metformin; MTC, medullary thyroid carcinoma; NYHA, New York Heart Association; s.c., subcutaneous; SU, sulphonylurea; T2D, type 2 diabetes; TZD, thiazolidinedione.
For Japanese participants, only those ≥20 years of age were included in the study.
Stable treatment was defined as having unchanged medication and dose (SUSTAIN 1–4) or ± 20% change in total daily dose (SUSTAIN 5) for at least 90 days prior to screening.
Except short‐term insulin treatment.
Per modification of diet in renal disease formula.
Semaglutide s.c. 0.5 and 1.0 mg doses were administered in all SUSTAIN trials except for SUSTAIN 3, in which only the 1.0 mg dose was administered. The two semaglutide maintenance doses were administered once weekly. Participants were followed throughout the planned treatment period, irrespective of treatment adherence. In all SUSTAIN trials, semaglutide‐treated participants followed a fixed dose‐escalation regimen. The semaglutide 0.5 mg maintenance dose was reached after 4 weeks of semaglutide 0.25 mg once weekly, and the semaglutide 1.0 mg maintenance dose was reached after 8 weeks: 4 weeks of semaglutide 0.25 mg once weekly, followed by 4 weeks of 0.5 mg once weekly.
Key prespecified endpoints were similar across the SUSTAIN 1 to 5 trials.12, 13, 14, 15, 16 For all trials, the primary endpoint was change in HbA1c from baseline to end of treatment (30 or 56 weeks); the confirmatory secondary endpoint was change in body weight from baseline to end of treatment (30 or 56 weeks). Data from SUSTAIN 617 were not included in the present analysis because of the different design of this trial compared with others in the SUSTAIN programme; the SUSTAIN 1 to 5 trials were efficacy trials that sought to assess the maximum treatment effects of semaglutide vs comparators, whereas SUSTAIN 6 was designed to assess the safety of semaglutide in people with T2D who were at high cardiovascular risk.
2.2. Study population
Key inclusion and exclusion criteria were generally comparable across trials. Participants in the SUSTAIN 1 to 5 trials were aged ≥18 years and diagnosed with T2D, and had baseline HbA1c <53 mmol/mol (7.0%) to 86 mmol/mol (10.0%) (SUSTAIN 1, 4 and 5) or <53 mmol/mol (7.0%) to 91 mmol/mol (10.5%).
The main exclusion criteria were history of chronic or idiopathic acute pancreatitis, known proliferative retinopathy or maculopathy requiring acute treatment, and baseline estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 (SUSTAIN 1, 4 and 5) or <60 mL/min/1.73 m2 (SUSTAIN 2 and 3).
The SUSTAIN 1 to 5 trials included participants at various stages of their diabetes treatment: drug‐naïve (SUSTAIN 1); on metformin and/or thiazolidinediones (SUSTAIN 2); on 1 to 2 OADs comprising either metformin, thiazolidinediones and/or sulphonylureas (SUSTAIN 3); on metformin with or without sulphonylureas (SUSTAIN 4); or on basal insulin with or without metformin (SUSTAIN 5; Table 1). The main between‐trial differences related to pre‐existing treatments, if any, at screening.12, 13, 14, 15, 16
All trials were conducted in compliance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.19 The protocols were approved by local ethics committees and institutional review boards. Written informed consent was obtained from all participants before trial commencement.
2.3. Study endpoints and assessments
The primary, prespecified, composite endpoint in the present analysis was the proportion of participants from all five trials achieving HbA1c <53 mmol/mol (7.0%) at the end of treatment (30 or 56 weeks), without weight gain and with no severe or BG‐confirmed symptomatic hypoglycaemia. The secondary, post hoc composite endpoint was the proportion of participants achieving the primary composite endpoint without moderate or severe GI AEs. Other endpoints included proportions of participants achieving HbA1c <53 mmol/mol (7.0%) with either no body weight gain, no moderate or severe GI AEs, no severe or BG‐confirmed hypoglycaemia, no severe hypoglycaemia, or no BG‐confirmed hypoglycaemia.
Severe or BG‐confirmed symptomatic hypoglycaemia was defined as an episode that is considered severe according to the American Diabetes Association classification,20 or BG‐confirmed by a plasma glucose value <3.1 mmol/L (56 mg/dL) with symptoms consistent with hypoglycaemia. No weight gain was defined as strictly no positive change from baseline in body weight at the end of treatment.
2.4. Analyses and statistical methods
The analyses reported in the present paper were performed on all randomized and treated participants (full analysis set) using on‐treatment data without rescue medication. To avoid potential confounding of initiation of antidiabetic rescue therapies on efficacy endpoints, data collected after initiation of antidiabetic rescue therapies were excluded.
Endpoints were analysed using separate logistic regression models, with treatment, trial‐specific stratification and country as fixed factors, and baseline values of HbA1c and body weight (where applicable) as covariates. Before analysis, missing HbA1c data were imputed from separate mixed models for repeated measurements, with treatment, trial‐specific stratification and country and parameter‐specific baseline value, all nested within visit.
3. RESULTS
3.1. Participants in the SUSTAIN 1 to 5 trials
Across the SUSTAIN 1 to 5 trials, 3918 participants with T2D were randomized to once‐weekly s.c. semaglutide 0.5, 1.0 mg, or comparators. A total of 3899 (>99%) were exposed to an investigational product and 3565 (>90%) participants completed the trials (Table 2). Discontinuation rates with semaglutide were generally consistent across SUSTAIN 1 to 5. In individual trials, discontinuation rates were higher with semaglutide vs placebo (SUSTAIN 1 and 5), sitagliptin (SUSTAIN 2) and insulin glargine (SUSTAIN 4; Table 2). Discontinuation rates were similar between semaglutide and exenatide ER in SUSTAIN 3 (Table 2). Rescue medication was administered in 99 semaglutide‐treated and 185 comparator‐treated participants (total of 284). The types of rescue medication administered are summarized in Table S1. Most participants requiring rescue medication received metformin, sulphonylureas or insulin. In the SUSTAIN 2 trial, more participants in the comparator arm received sulphonylureas than in the semaglutide arms. In both the SUSTAIN 2 and 5 trials, more participants in the comparator arm initiated insulin or intensified their existing background insulin as rescue medication than in the semaglutide arms.
Table 2.
Participant disposition and baseline characteristics in the SUSTAIN 1 to 5 trials
| SUSTAIN 1: Semaglutide vs placebo 30 weeks | SUSTAIN 2: Semaglutide vs sitagliptin 100 mg 56 weeks | SUSTAIN 3: Semaglutide vs exenatide ER 2.0 mg 56 weeks | SUSTAIN 4: Semaglutide vs IGlar 30 weeks | SUSTAIN 5: Semaglutide add‐on to insulin vs placebo 30 weeks | |
|---|---|---|---|---|---|
| Participant disposition, N (%) | |||||
| Randomized | 388 | 1231 | 813 | 1089 | 397 |
| Exposed | 387 (99.7) | 1225 (99.5) | 809 (99.5) | 1082 (99.4) | 396 (99.7) |
| Trial completers | 359 (92.5) | 1163 (94.5) | 743 (91.4) | 1020 (93.7) | 380 (95.7) |
| Premature treatment discontinuation | |||||
| Semaglutide 0.5 mg | 17 (13.3) | 53 (13.0) | N/A | 49 (13.5) | 14 (10.6) |
| Semaglutide 1.0 mg | 16 (12.3) | 61 (14.9) | 82 (20.3) | 55 (15.3) | 16 (12.2) |
| Comparator | 14 (10.9) | 32 (7.9) | 85 (21.0) | 26 (7.2) | 13 (9.8) |
| Participants administered rescue medication | |||||
| Semaglutide 0.5 mg | 6 (4.7) | 25 (6.1) | N/A | 14 (3.9) | 3 (2.3) |
| Semaglutide 1.0 mg | 6 (4.6) | 10 (2.4) | 29 (7.2) | 9 (2.5) | 1 (0.8) |
| Comparator | 27 (20.9) | 85 (20.9) | 48 (11.9) | 5 (1.4) | 21 (15.8) |
| Baseline characteristics, mean (SD) | |||||
| Age, y | 53.7 (11.3) | 55.1 (10.0) | 56.6 (10.7) | 56.5 (10.4) | 58.8 (10.1) |
| Male gender, % | 54.3 | 50.6 | 55.3 | 53.0 | 56.1 |
| Diabetes duration, years | 4.2 (5.5) | 6.6 (5.1) | 9.2 (6.3) | 8.6 (6.3) | 13.3 (7.8) |
| Body weight, kg | 91.9 (23.8) | 89.5 (20.3) | 95.8 (21.5) | 93.5 (21.8) | 91.7 (21.0) |
| BMI, kg/m2 | 32.9 (7.7) | 32.5 (6.2) | 33.8 (6.7) | 33.0 (6.5) | 32.2 (6.2) |
| HbA1c, % | 8.1 (0.9) | 8.1 (0.9) | 8.3 (1.0) | 8.2 (0.9) | 8.4 (0.8) |
| HbA1c, mmol/mol | 64.5 (9.3) | 64.8 (10.1) | 67.7 (10.4) | 65.8 (9.7) | 67.9 (9.2) |
| FPG, mg/dL | 175.7 (48.2) | 169.4 (40.7) | 189.0 (48.7) | 175.3 (51.2) | 155.9 (53.7) |
| FPG, mmol/L | 9.7 (2.7) | 9.4 (2.3) | 10.5 (2.7) | 9.7 (2.8) | 8.6 (3.0) |
| PPG incrementa, mg/dL | 45.3 (39.2) | 51.0 (37.6) | 39.7 (33.5) | 43.4 (35.1) | 55.5 (43.1) |
| PPG incrementa, mmol/L | 2.5 (2.2) | 2.8 (2.1) | 2.2 (1.9) | 2.4 (1.9) | 3.1 (2.4) |
Abbreviations: BMI, body mass index; ER, extended release; FPG, fasting plasma glucose; IGlar, insulin glargine; N, number of participants; PPG, postprandial glucose
PPG increment based on the 7‐point (SUSTAIN 1, 2, 3, 5) or 8‐point (SUSTAIN 4) self‐measured blood glucose profile. Trial completer refers to those participants who attended the follow‐up visit.
Overall, participant baseline characteristics within trials were similar across treatment groups (Table 2), with any differences among trials reflecting the eligibility criteria. There were differences among the trials in diabetes duration, indicative of the varying intensity of antidiabetic treatments received by the participants with T2D.
3.2. Primary composite endpoint
The proportion of participants achieving the prespecified primary composite endpoint of HbA1c <53 mmol/mol (7.0%) at end of treatment without weight gain and with no severe or BG‐confirmed symptomatic hypoglycaemia was 47% to 66% with semaglutide 0.5 mg and 57% to 74% with semaglutide 1.0 mg, vs 7% to 19% with placebo and 16% to 29% with active comparators (Figure 1). Significantly more participants treated with semaglutide 0.5 and 1.0 mg once weekly achieved this composite endpoint vs comparators (all P < .0001; Figure 1).
Figure 1.
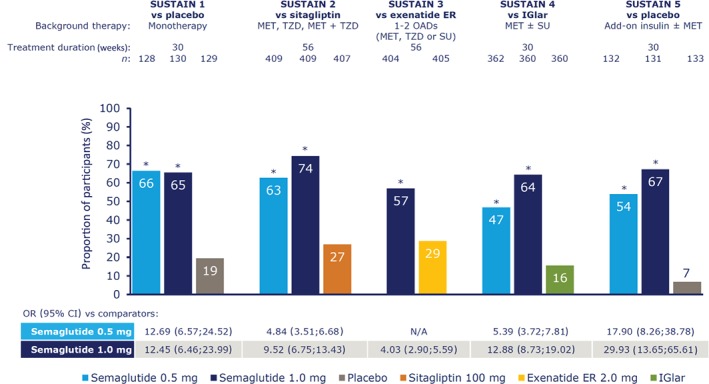
Participants achieving glycated haemoglobin (HbA1c) <53 mmol/mol (7.0%) without weight gain and with no severe or blood glucose‐confirmed symptomatic hypoglycaemia. *P < .0001 vs comparator. On‐treatment without rescue medication data. The binary endpoint was analysed using a logistic regression with treatment, trial‐specific stratification and country as fixed factors, and baseline HbA1c and body weight as covariates. Missing data were imputed from a mixed model for repeated measurements for change from baseline in which post‐baseline data were analysed, with treatment, trial‐specific stratification and country as fixed factors, and baseline value as covariate, all nested within visit. CI, confidence interval; ER, extended release; IGlar, insulin glargine; MET, metformin; n, number of participants contributing to analysis; N/A, not applicable; OAD, oral antidiabetic drug; OR, odds ratio; SU, sulphonylurea; TZD, thiazolidinedione
3.3. Post hoc composite endpoint
In the post hoc analysis, the proportion of participants achieving the composite endpoint of HbA1c <53 mmol/mol (7.0%) at end of treatment without weight gain, with no severe or BG‐confirmed symptomatic hypoglycaemia, and with no moderate or severe GI AEs was 40% to 55% with semaglutide 0.5 mg and 46% to 64% with semaglutide 1.0 mg, vs 7% to 19% with placebo and 14% to 25% with active comparators (Figure 2). Significantly more semaglutide‐treated participants achieved this composite endpoint vs comparators (all P < .0001; Figure 2).
Figure 2.
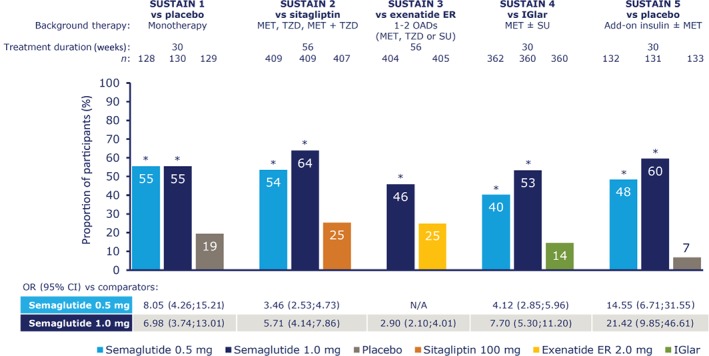
Participants achieving glycated haemoglobin (HbA1c) <53 mmol/mol (7.0%) without weight gain, with no severe or blood glucose‐confirmed symptomatic hypoglycaemia, and with no moderate or severe gastrointestinal adverse events. *P < .0001 vs comparator. On‐treatment without rescue medication data. The binary endpoint was analysed using a logistic regression with treatment, trial‐specific stratification, and country as fixed factors and baseline HbA1c and body weight as covariates. Missing data were imputed from a mixed model for repeated measurements for change from baseline in which post‐baseline data were analysed with treatment, trial‐specific stratification and country as fixed factors, and baseline value as covariate, all nested within visit. CI, confidence interval; ER, extended release; IGlar, insulin glargine; MET, metformin; n, number of participants contributing to analyses; N/A, not applicable; OAD, oral antidiabetic drug; OR, odds ratio; SU, sulphonylurea; TZD, thiazolidinedione
3.4. Analyses of individual components of composite endpoints
Results for the individual components of composite endpoints were mostly consistent with the findings for the primary and secondary composite endpoints. In SUSTAIN 1 to 5, the proportion of participants achieving HbA1c <53 mmol/mol (7.0%) was 58% to 74% with semaglutide 0.5 mg and 67% to 79% with semaglutide 1.0 mg, vs 11% to 25% with placebo and 36% to 40% with active comparators (Figure 3A). Significantly more participants achieved HbA1c targets of <53 mmol/mol (7.0%) with semaglutide 0.5 and 1.0 mg than with comparators (all P < .0001; Figure 3A). The proportion of participants with no weight gain was 82% to 89% with semaglutide 0.5 mg and 86% to 97% with semaglutide 1.0 mg, vs 64% to 66% with placebo and 34% to 69% with active comparators (all P ≤ .0006; Figure 3B). The proportion of participants with no severe or BG‐confirmed symptomatic hypoglycaemia was 92% to 100% with semaglutide 0.5 mg and 89% to 100% with semaglutide 1.0 mg, vs 95% to 100% with placebo and 89% to 99% with active comparators (P < .008 for semaglutide 0.5 and 1.0 mg vs IGlar in SUSTAIN 4; Figure 3C).
Figure 3.
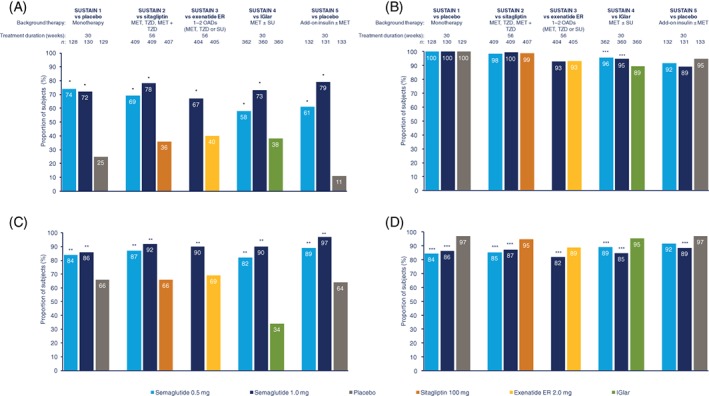
Proportion of participants achieving glycated haemoglobin (HbA1c) <53 mmol/mol (7.0%) (A), with no weight gain (B), no severe or BG‐confirmed symptomatic hypoglycaemia (C) or no moderate or severe gastrointestinal adverse events (D). *P < .0001; **P < .001; ***P < .01 vs comparator. On‐treatment without rescue medication data. The binary endpoint was analysed using a logistic regression with treatment, trial‐specific stratification and country as fixed factors, and baseline HbA1c and body weight as covariates. Missing data were imputed from a mixed model for repeated measurements for change from baseline in which post‐baseline data were analysed with treatment, trial‐specific stratification and country as fixed factors, and baseline value as covariate, all nested within visit. ER, extended release; IGlar, insulin glargine; MET, metformin; n, number of participants contributing to analysis; OAD, oral antidiabetic drug; SU, sulphonylurea; TZD, thiazolidinedione
The proportion of participants with no moderate or severe GI AEs was lower with semaglutide than with comparators. The proportion of participants with no moderate/severe GI AEs was 84% to 92% with semaglutide 0.5 mg and 82% to 89% with semaglutide 1.0 mg, vs 97% with placebo and 89% to 95% with active comparators (P < 0.01 for all comparisons except semaglutide 0.5 mg vs placebo in SUSTAIN 5; Figure 3D).
The proportion of participants achieving HbA1c <53 mmol/mol (7.0%) with no body weight gain ranged from 49% to 66% with semaglutide 0.5 mg, and 62% to 77% with semaglutide 1.0 mg, vs 8% to 19% with placebo and 17% to 31% with active comparators. Significantly more participants achieved HbA1c targets of <53 mmol/mol (7.0%) and no weight gain with semaglutide 0.5 and 1.0 mg than with comparators (P < .0001 for all comparisons; Figure S1A). The proportion of participants achieving HbA1c of <53 mmol/mol (7.0%) with no moderate or severe GI AEs was 51% to 62% with semaglutide 0.5 mg, and 54% to 69% with semaglutide 1.0 mg, vs 10% to 25% with placebo and 34% to 36% with active comparators (P ≤ .0002 for all comparisons; Figure S1B).
The proportion of participants achieving HbA1c <53 mmol/mol (7.0%) without severe or BG‐confirmed hypoglycaemia was 54% to 74% with semaglutide 0.5 mg, and 61% to 78% with semaglutide 1.0 mg, vs 10% to 25% with placebo and 34% to 37% with active comparators (P < .0001 for all comparisons; Figure S1C). The proportion of participants achieving HbA1c <53 mmol/mol (7.0%) with no severe hypoglycaemia (as defined by the American Diabetes Association) ranged from 57% to 74% with semaglutide 0.5 mg, 67% to 78% with semaglutide 1.0 mg, vs 11% to 25% with placebo and 36% to 40% with comparators (P < .0001 for all comparisons; Figure S2A). The proportion of participants achieving HbA1c <53 mmol/mol (7.0%) with no BG‐confirmed hypoglycaemia was 54% to 74% with semaglutide 0.5 mg, 61% to 78% with semaglutide 1.0 mg, vs 10% to 25% with placebo and 34% to 37% with active comparators (P < .0001 for all comparisons; Figure S2B).
4. DISCUSSION
When attempting to achieve adequate glycaemic control, it is important to also consider the risk of unwanted outcomes such as hypoglycaemia and weight gain.1, 2, 3, 4, 8 The present analysis of combined data from the SUSTAIN clinical trial programme evaluated the proportion of participants who achieved the HbA1c target of <53 mmol/mol (7.0%) with no weight gain, no severe or BG‐confirmed symptomatic hypoglycaemia, and no moderate or severe GI AEs. The cumulative sample size of >3900 participants represents a diverse population of participants with T2D.
Across the SUSTAIN 1 to 5 trials, approximately half to two‐thirds of participants who received semaglutide 0.5 mg achieved an HbA1c concentration < 53 mmol/mol (7.0%) without weight gain and with no severe or BG‐confirmed symptomatic hypoglycaemia. Up to three‐quarters of participants achieved this outcome with semaglutide 1.0 mg. With both semaglutide doses, significantly more participants achieved this composite endpoint vs those receiving comparators.
The overall difference was mostly driven by larger proportions of participants achieving HbA1c <53 mmol/mol (7.0%) as well as by the proportions of participants experiencing no weight gain. The proportions of participants achieving HbA1c <53 mmol/mol (7.0%) with only one component of “no body weight gain”, “no moderate or severe GI AEs”, or “no severe or BG‐confirmed symptomatic hypoglycaemia”, were each significantly greater with semaglutide 0.5 and 1.0 mg vs comparators. The proportions of participants receiving semaglutide achieving HbA1c <53 mmol/mol (7.0%) with no hypoglycaemia were consistent regardless of the definition of hypoglycaemia used (severe, BG‐confirmed symptomatic, or severe or BG‐confirmed symptomatic).
These findings are generally consistent with those from other studies and compare favourably with published data for other GLP‐1RAs.1, 6, 7, 21 For instance, a meta‐analysis of seven clinical trials in participants with T2D showed that 32% to 40% of participants receiving liraglutide achieved HbA1c <53 mmol/mol (7.0%) concentrations without weight gain or hypoglycaemia, vs 8% for placebo and 8% to 25% for comparators (exenatide, IGlar, sitagliptin, sulphonylureas or thiazolidinedione).21 A similar analysis of the AWARD clinical trial programme showed that participants with T2D receiving dulaglutide were equally likely to achieve HbA1c <53 mmol/mol (7.0%) without weight gain or hypoglycaemia as those receiving liraglutide.22 Furthermore, the SUSTAIN 7 trial showed that participants with T2D who were treated with once‐weekly semaglutide experienced superior reductions in HbA1c and body weight, in comparison with those treated with dulaglutide.18
Across the SUSTAIN 1 to 5 trials, semaglutide was well tolerated, with a similar safety profile to that of other GLP‐1RAs.12, 13, 14, 15, 16, 23, 24 The most common AEs leading to discontinuation of semaglutide were GI in nature and, overall, most cases of GI AEs were mild or moderate and of a short duration.12, 13, 14, 15, 16, 23, 24 GI AEs are commonly observed across the GLP‐1RA class,2, 9 and may affect treatment outcomes in the real‐world setting. In SUSTAIN 1 to 5, >80% of participants experienced no GI AEs, regardless of their randomized treatment; however, the proportion of participants with no GI AEs was lower with semaglutide, vs comparators, except in the semaglutide 0.5 mg group in SUSTAIN 5. With semaglutide 0.5 or 1.0 mg, approximately half of the participants achieved HbA1c concentrations <53 mmol/mol (7.0%) without weight gain, severe or BG‐confirmed symptomatic hypoglycaemia, or moderate/severe GI AEs, which was significantly more than participants treated with comparators. The overall effect for the post hoc composite endpoint was mostly driven by the proportion of participants achieving HbA1c <53 mmol/mol (7.0%) and those experiencing no weight gain. Similar analyses with liraglutide and dulaglutide have not examined the role of GI AEs in participants achieving HbA1c <53 mmol/mol (7.0%) without weight gain or severe or BG‐confirmed symptomatic hypoglycaemia.21, 22
Both semaglutide 0.5 and 1.0 mg have demonstrated consistently superior efficacy in terms of glycaemic control. Treatment with semaglutide 0.5 or 1.0 mg has also previously been shown to result in superior weight reduction, from baseline values, vs comparators.12, 13, 14, 15, 16 This effect was generally greater with semaglutide 1.0 than with 0.5 mg.
A potential criticism of the present analyses of the SUSTAIN 1 to 5 trials concerns the analyses of individual components of the prespecified and post hoc composite endpoints. When evaluated in isolation, each component may differ in their clinical importance; therefore, one should consider the validity of giving each component equal weight when combining them into composite endpoints. Conversely, a clear advantage of the present analyses is the number of participants involved in the SUSTAIN 1 to 5 trials. The trials involved >3900 participants with T2D, and various stages of the treatment and the disease were represented, from treatment‐naïve participants receiving monotherapy to those on insulin and other background therapies. Furthermore, each trial independently recruited sufficient participants to allow meaningful evaluations between semaglutide and a number of relevant comparators. Trials varied in duration (30 weeks for SUSTAIN 1, 4 and 5; 56 weeks for SUSTAIN 2 and 3), which may potentially have affected the proportions of participants achieving the composite endpoint; however, with the 56‐week trials, large proportions of participants achieved both composite endpoints, suggesting that the effects of semaglutide are sustainable over the long term.
Overall, these results indicate a consistency in the effect of semaglutide 0.5 and 1.0 mg across a broad range of participants with T2D, including drug‐naïve participants and those on background therapies consisting of OADs and/or insulin.
In conclusion, across the overall populations with T2D participating in the SUSTAIN 1 to 5 trials, significantly more participants achieved the HbA1c target of <53 mmol/mol (7.0%) without weight gain and with no hypoglycaemia when treated with semaglutide than with comparators. More participants benefitted from treatment with semaglutide vs comparators even when the composite endpoint was expanded to include the absence of moderate or severe GI AEs as a component. Semaglutide helped more people with T2D achieve HbA1c targets vs comparators in the SUSTAIN 1 to 5 trials, while avoiding unwanted outcomes such as weight gain, hypoglycaemia, and GI side effects.
Supporting information
Table S1 Types of rescue medications administered across SUSTAIN 1–5.
Figure S1 Proportion of subjects achieving HbA1c < 7.0% with no weight gain (A), HbA1c < 7.0% with no moderate or severe GI AEs (B), or HbA1c < 7.0% with no severe or BG‐confirmed symptomatic hypoglycaemia (C).
Figure S2 Proportion of subjects achieving HbA1c < 7.0% with no severe symptomatic hypoglycaemia (A) or HbA1c < 7.0% with no BG‐confirmed symptomatic hypoglycaemia (B).
ACKNOWLEDGMENTS
We thank all the participants, investigators and trial‐site staff who were involved in the conduct of the trials. We also thank Hrvoje Vrazic, MD (Novo Nordisk), for his review and input into the manuscript. Haydn Liang, PhD (AXON Communications), provided medical writing and editorial assistance and received compensation from Novo Nordisk.
Conflict of interest
SB and VW received research grants from Novo Nordisk for the current study. CD received non‐financial support from Novo Nordisk for the current study. Outside the submitted work, JHD received personal fees from Novo Nordisk; CD received grants and personal fees from Novo Nordisk and grants from the National Institutes of Health, Theracos, Sanofi and Janssen; SB received personal fees from Eli Lilly, MSD, AstraZeneca, Boehringer Ingelheim, Sanofi Aventis, Novo Nordisk, and Janssen; VW received grants and personal fees from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, AstraZeneca and Sanofi. JU participates at an advisory board for Novo Nordisk. OKHH and JZ are employees of Novo Nordisk. JZ is a shareholder of Novo Nordisk.
Author contributions
JHD and JZ contributed to the study design; CD, JZ and VW contributed to the collection and/or handling of data; JHD, CD, SB, OKHH, JZ and VW contributed to the analysis and/or interpretation of data; literature searches were conducted by JZ; JHD, CD, SB, JU, JZ and VW contributed to the writing, reviewing, and/or editing of the manuscript.
DeVries JH, Desouza C, Bellary S, et al. Achieving glycaemic control without weight gain, hypoglycaemia, or gastrointestinal adverse events in type 2 diabetes in the SUSTAIN clinical trial programme. Diabetes Obes Metab. 2018;20:2426–2434. 10.1111/dom.13396
Funding information Novo Nordisk A/S, Søborg, Denmark
REFERENCES
- 1. American Diabetes Association . Standards of medical care in diabetes‐2017. Diabetes Care. 2017;40(Suppl 1):S1‐S135.27979885 [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetologia. 2015;58(3):429‐442. [DOI] [PubMed] [Google Scholar]
- 3. Bailey CJ, Blonde L, Del Prato S, Leiter LA, Nesto R. What are the practical implications for treating diabetes in light of recent evidence? Updated recommendations from the global partnership for effective diabetes management. Diab Vasc Dis Res. 2009;6(4):283‐287. [DOI] [PubMed] [Google Scholar]
- 4. Bailey CJ, Aschner P, Del Prato S, et al. Individualized glycaemic targets and pharmacotherapy in type 2 diabetes. Diab Vasc Dis Res. 2013;10(5):397‐409. [DOI] [PubMed] [Google Scholar]
- 5. Kieffer TJ, Habener JF. The glucagon‐like peptides. Endocr Rev. 1999;20(6):876‐913. [DOI] [PubMed] [Google Scholar]
- 6. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med. 2011;154(9):602‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ostawal A, Mocevic E, Kragh N, Xu W. Clinical effectiveness of liraglutide in type 2 diabetes treatment in the real‐world setting: a systematic literature review. Diabetes Ther. 2016;7(3):411‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58(18):7370‐7380. [DOI] [PubMed] [Google Scholar]
- 11. Marbury T, Flint A, Segel S, Lindegaard M, Lasseter K. Pharmacokinetics and tolerability of a single dose of semaglutide, a once‐weekly human GLP‐1 analog, in subjects with and without renal impairment. Clin Pharmacokinet. 2017;56(11):1381‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 13. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 14. Ahmann AJ, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2017;41(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 15. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 16. Rodbard H, Lingvay I, Reed J, et al. Efficacy and safety of semaglutide once‐weekly vs placebo as add‐on to basal insulin alone or in combination with metformin in subjects with type 2 diabetes (SUSTAIN 5). Diabetologia. 2016;59(Suppl 1):S364‐S365. [Google Scholar]
- 17. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 18. Pratley R, Aroda VR, Lingvay I, et al. Semaglutide once weekly versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 19. World Medical Association Declaration Of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925‐926. [PubMed] [Google Scholar]
- 20. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zinman B, Schmidt WE, Moses A, Lund N, Gough S. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta‐analysis of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012;14(1):77‐82. [DOI] [PubMed] [Google Scholar]
- 22. Dungan KM, Raz I, Skrivanek Z, Sealls W, Fahrbach JL. Achieving the composite endpoint of glycated haemoglobin <7.0%, no weight gain and no hypoglycaemia in the once‐weekly dulaglutide AWARD programme. Diabetes Obes Metab. 2016;18(1):49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION‐3): 3‐year results of an open‐label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464‐473. [DOI] [PubMed] [Google Scholar]
- 24. Russell‐Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Types of rescue medications administered across SUSTAIN 1–5.
Figure S1 Proportion of subjects achieving HbA1c < 7.0% with no weight gain (A), HbA1c < 7.0% with no moderate or severe GI AEs (B), or HbA1c < 7.0% with no severe or BG‐confirmed symptomatic hypoglycaemia (C).
Figure S2 Proportion of subjects achieving HbA1c < 7.0% with no severe symptomatic hypoglycaemia (A) or HbA1c < 7.0% with no BG‐confirmed symptomatic hypoglycaemia (B).


