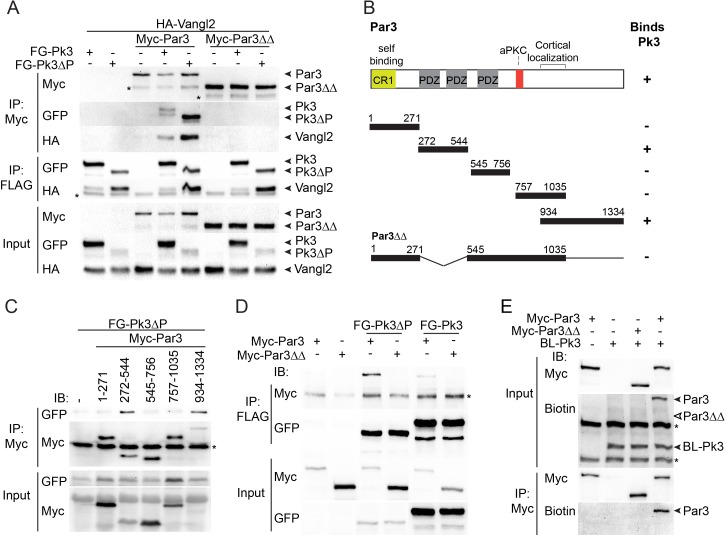
Figure 4. The association of Par3 with the Pk3/Vangl2 complex and the identification of Pk3-interacting domains.
(A) HEK293T cells were transfected with Par3, Pk3 or Pk3∆PET (Pk3∆P) and Vangl2 constructs as indicated. Sequential pulldowns of protein complexes containing Myc-Par3, FLAG-GFP(FG)-Pk3 or FG-Pk3∆P and HA-Vangl2 with Myc-Trap and anti-FLAG (M2) beads are shown. Note that Par3 binds Vangl2 only in the presence of Pk3 or Pk3∆P. Asterisks mark nonspecific bands. (B). Schematic showing the Par3 constructs used in these experiments and the summary of Par3 binding. (C) Co-immunoprecipitation of Myc-Par3 constructs with FG-Pk3∆P (see Figure 3 legend). (A sterisk indicates IgG heavy chain. (D) Pulldowns of FG-Pk3∆P or wild type FG-Pk3 with Myc-Par3 or Myc-Par3∆∆. Asterisk shows a nonspecific band. (E) Interaction of Par3 and Pk3 assessed by proximity biotinylation in Xenopus embryos. Exogenous Par3 but not Par3∆∆ is biotinylated by BL-Pk3. Black arrowheads point to biotinylated Par3 and BL-Pk3, and white arrowheads indicate the expected position of Par3∆∆. Asterisks indicate endogenous proteins detected by anti-biotin antibodies. Protein levels are shown by immunoblotting with anti-Myc, anti-HA, anti-GFP and anti-biotin antibodies as indicated.