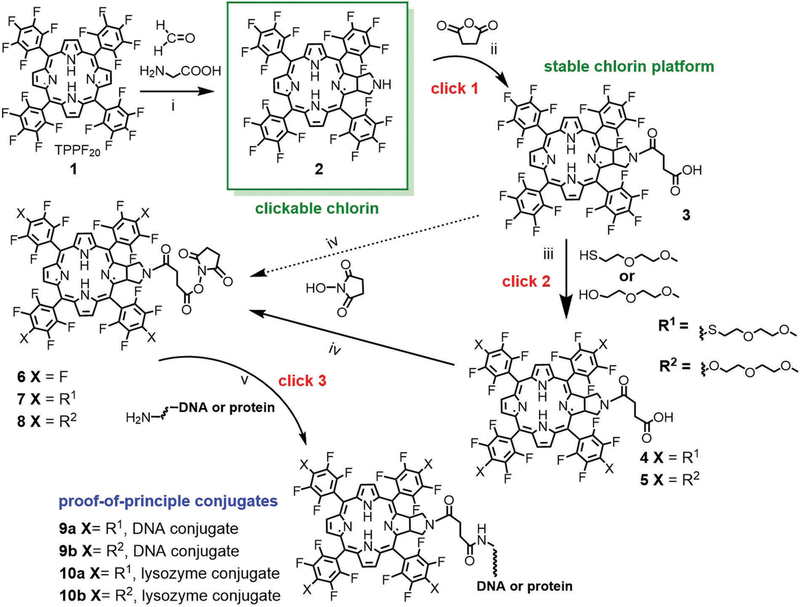
Scheme 2.
(i) Glycine/paraformaldehyde (1 : 1), added in four aliquots in chlorobenzene, 145 °C, 8 h, 36% yield. (ii) 5 eq. succinic anhydride, CH2Cl2, NEt3, 25 °C, overnight, 95% yield. (iii) For HS-PEG, 4.5 eq. 2-(2-methoxyethoxy)ethane thiol and stoichiometric K2CO3 in acetone, 25 °C, 6 h, 85%; for HO-PEG 10 eq. 2-(2-methoxyethoxy)ethanol and stoichiometric K2CO3 in dry acetone, reflux, overnight, 85%. (iv) 1.2 eq. N-hydroxysuccinimide in dioxane or tetrahydrofuran with 1.2 eq. dicyclohexylcarbodiimide at 25 °C, overnight, quantitative yield. (v) Bio-conjugation depends on the specific targeting molecule but is generally accomplished in buffer or DMSO at 25 °C for 2–10 h. Chlorins were reacted in a 5 μM solution of a 14 nt DNA with an amino terminated tether (10 : 1 ratio; 5’-NH2-(CH2)6-NH-TTCTTCTCCTTTCT-3’), pH 7.4 phosphate buffer overnight, 37 °C. For the lysozyme (hydrolases) conjugate, a 100 μM solution of 8 was allowed to react with lysozyme in DMSO over night at room temperature and purified by gel filtration on a 5 mL column of Sephadex G-25 with water as eluent.