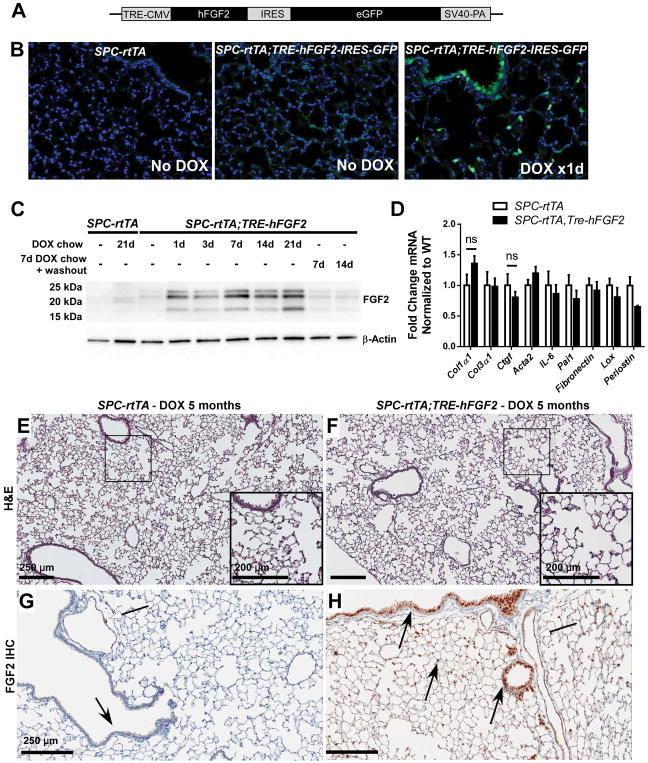
Figure 1.
Doxycycline-inducible and reversible overexpression of human FGF2 in mouse lung. (A) Diagram of the TRE-hFGF2-IRES-GFP construct used to generate transgenic TRE-hFGF2 mice. (B) SPC-rtTA and double-transgenic SPC-rtTA;TRE-hFGF2-IRES-GFP mice were fed either normal chow (No DOX) or doxycycline chow (DOX), and lungs were collected for frozen sections. GFP was directly visualized with DAPI as a nuclear stain. (C) SPC-rtTA and double-transgenic SPC-rtTA;TRE-hFGF2-IRES-GFP (or SPC-rtTA;TRE-hFGF2) mice were fed normal chow or DOX chow for specified times and whole lung protein lysates were immunoblotted for FGF2. Where indicated, mice were fed DOX chow for 7 days followed by doxycycline washout with normal chow for 7 or 14 days. (D) Quantitative RT-PCR analysis of whole-lung RNA from DTG and STG control mice fed DOX chow for 21 days. ΔCt values were normalized to Gapdh and expressed as fold change from STG controls. “ns” = not significant. (E–H) SPC-rtTA and SPC-rtTA;TRE-hFGF2 mice were fed DOX chow for 5 months, and lungs were collected for H&E staining (E, F), and FGF2 immunohistochemistry (G, H). White-headed arrows indicate FGF2 staining in vascular smooth muscle; black-headed arrows indicate FGF2 staining in airway and alveolar epithelial cells, which is increased in SPC-rtTA;TRE-hFGF2 mice. Higher magnification of marked areas in E and F is shown in insets, scale bar = 250 μM (200 μM in insets).