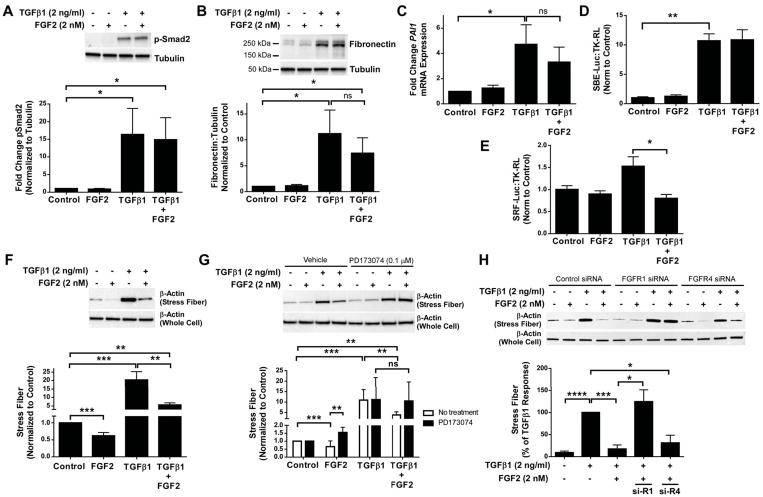
Figure 5.
Recombinant FGF2 inhibits TGFβ1-induced stress fiber formation and activation of the SRF pathway. Primary HLFs were serum-starved and treated with FGF2 (2 nM), TGFβ1 (2 ng/ml), or FGF2 + TGFβ1 for 1 hour or 48 hours. (A) Total protein was collected at 1 hour and immunoblotting was performed for phosphorylated Smad2, normalized to tubulin, and expressed as fold change from untreated control. (B) Total protein was collected at 48 hours and immunoblotting was performed for fibronectin and tubulin. Densitometry was performed for fibronectin, normalized to tubulin, and expressed as fold change from untreated control. (C) Total RNA was collected at 48 hours, and qRT-PCR was performed for PAI1. ΔCt values were normalized to GAPDH and expressed as fold change from untreated controls. (D, E) Primary HLFs were transfected with SBE-luciferase and TK-renilla luciferase (D) or SRF-luciferase and TK-renilla luciferase (E), serum-starved overnight, and treated with FGF2 (2 nM), TGFβ1 (2 ng/ml), or FGF2 + TGFβ1 for 24 hours. Firefly luciferase activity was measured and normalized to renilla luciferase, and expressed as fold change from untreated controls. (F, G) Primary HLFs were treated with FGF2, TGFβ1, or FGF2 + TGFβ1 ± PD173074 for 48 hours prior to collection of purified stress fibers and total cell lysates. (H) HLFs were transfected with control siRNA, FGFR1 siRNA, or FGFR4 siRNA, and treated with FGF2, TGFβ1, or TGFβ1 + FGF2 for 48 hours prior to collection of purified stress fibers and total cell lysates. Western blot for β-actin was performed on stress fiber preparations and whole cell lysates. Densitometry for stress fiber β-actin was normalized to total cell lysate β-actin, and expressed as fold change from untreated control. “ns” = not significant. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.005 using an unpaired 2-way t-test.