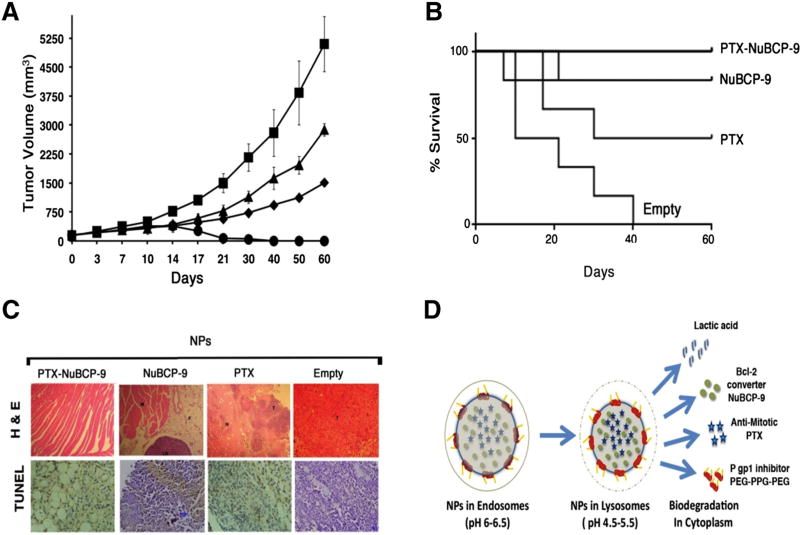
Figure 7.
Antitumor activity of PTX-NuBCP-9/NPs. (A) BALB/c mice (6 per group) with subcutaneous Ehrlich tumors (~150 mm3) were treated IP with empty NPs (Squares), 10 mg/kg NuBCP-9 encapsulated NPs (IP, triangles, twice weekly), 10 mg/kg PTX encapsulated NPs (IP, diamonds, twice weekly) or 10 mg/kg PTX-NuBCP9 dual drug encapsulated NPs (IP, circles, twice weekly) for 3 weeks. Tumor measurements were performed on the indicated days. The results are expressed as tumor volumes (mean ± SD). (B) The results are expressed as the percentage survival as determined by Kaplan–Meier analysis for mice treated with empty NPs, NuBCP-9/NPs, PTX/NPs and PTX/NuBCP-9/NPs. The statistical analysis was performed between the empty NP control and the PTX-NuBCP-9/NP-treated group (P < 0.001). (C) Histopathology of tumor tissues obtained from mice treated with the empty NPs, PTX/NPs, NuBCP-9/NPs and PTX-NuBCP-9/NPs for 21 days and stained with hematoxylin and eosin (X400). (D) Schematic representation of the endosomal lyposomal cytoplasmic pH-dependent degradation of NPs and the release of different payloads.