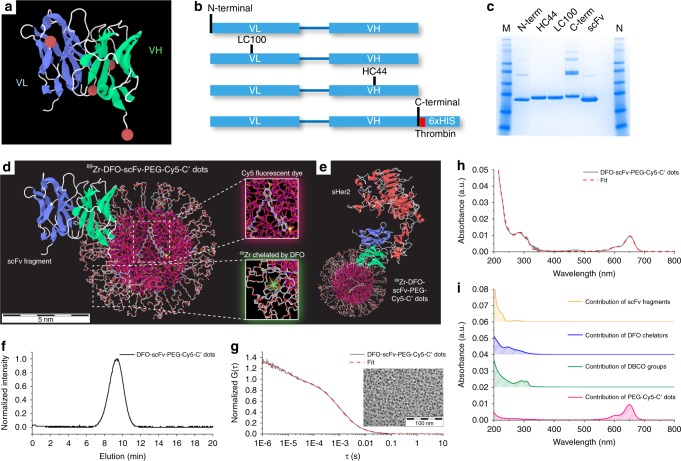
Fig. 1.
Site-specific engineering of anti-HER scFv fragments and synthesis of DFO-scFv-PEG-Cy5-C’ dots. a Three-dimensional (3D) scFv model highlighting sites of conjugation (i.e., red balls). b Schematic representation of scFvs directed to HER2/neu extracellular domain with sites of nnAA integration indicated (N-terminal, LC100, HC44, and C-terminal). Sites were rationally designed to be distal to the antigen binding domains (blue ribbon) and surface-exposed to avoid functionally important domains. c Affinity purified scFvs containing a nnAA at the indicated positions by SDS-PAGE under non-reducing conditions; HC44 and LC100 constructs generated mostly unique bands. scFv constructs with N-terminal, and in particular with C-terminal, nnAA produced disulfide-linked multimers observable as discrete bands of increasing molar mass. Unmodified scFv was resolved for comparison. d, e 3D rendering of 89Zr-DFO-scFv-PEG-Cy5-C’ dot (d) and 89Zr-DFO-scFv-PEG-Cy5-C’ dot bonded to sHER2 (e). The atoms of silicon, oxygen, carbon, nitrogen, sulfur, and zirconium in the 3D renderings are colored in purple, red, gray, blue, yellow, and light green, respectively. Hydrogen atoms are not displayed for better visualization. f–i GPC elugram (f), FCS correlation curve with fit (g), and UV–vis absorbance spectrum with fit (h) of DFO-scFv-PEG-Cy5-C’ dots. i Deconvolution of the UV–vis spectrum (h) into contributions of individual functional moieties, including PEG-Cy5-C’ dot (red), DBCO (green), DFO (blue), and scFv (orange). A representative TEM image of DFO-scFv-PEG-Cy5-C’ dots is shown in the inset of g