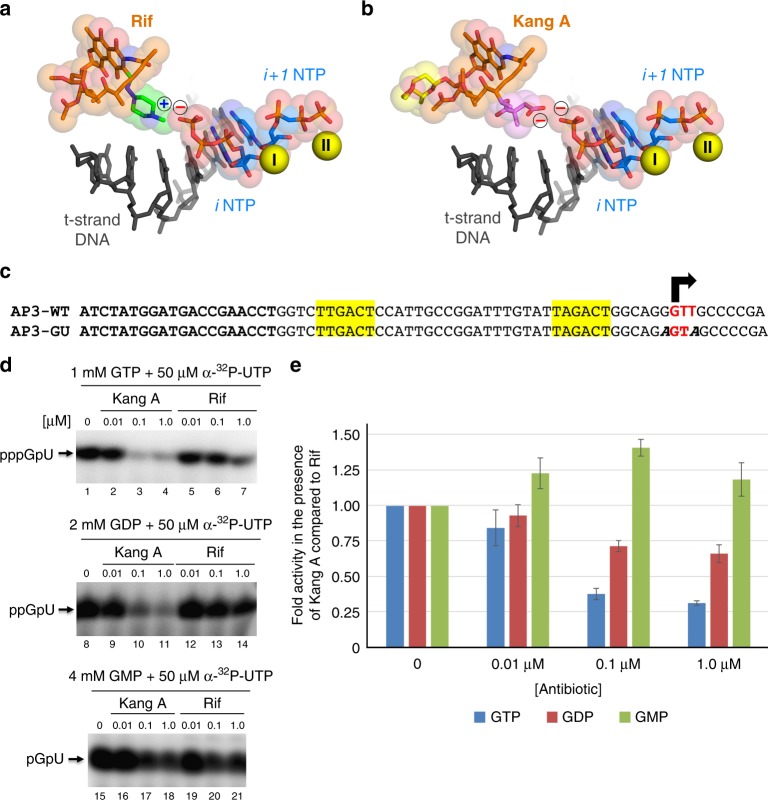
Fig. 6.
Structural basis for Kang A inhibition of iNTP binding. a View of the RNAP active site from the T. thermophilus de novo initiation complex (4Q4Z)50 with bound Rif superimposed. Shown is the t-strand DNA from +1 to −5 (dark gray), the initiating NTP substrates (i site NTP, ATP; i + 1 NTP, CMPCPP; blue carbon atoms) and two Mg2+-ions (yellow spheres; Mg2+I is the Mg2+-ion chelated in the RNAP active site, Mg2+II is bound to the i + 1 NTP). Rif is color-coded as in Fig. 4b. Rif and the NTPs are also shown with transparent van der Waals surfaces. The blue “+” denotes the positive charge of the Rif piperazine moiety, while the red “−” denotes the negative charge of the iNTP γ-phosphate. b Same as (A) but showing Kang A (colored as in Fig. 4c). The negative charge of K-acid is brought in close proximity to the negative charge of the iNTP γ-phosphate. c Sequence of AP3-GU promoter template used in in vitro abortive initiation assays monitoring the effect of Kang A or Rif on RNA dinucleotide synthesis with GTP, GDP, or GMP as the 5′-initiating nucleotide. The initial transcribed sequence of the Mtb AP3 promoter (top) was engineered to allow only RNA dinucleotide synthesis (5′-GU-3’) in the presence of GTP, GDP, or GMP as the 5′-initiating nucleotide and UTP. The mutated bases are denoted in bold italic (AP3-GU, bottom). d Kang A or Rif inhibition of in vitro abortive initiation of RNA dinucleotide synthesis using the AP3-GU promoter template; (top) 1 mM GTP + 50 μM α-32P-UTP; (middle) 2 mM GDP + 50 μM α-32P-UTP; (bottom) 4 mM GMP + 50 μM α-32P-UTP. e Plotted is the RNA dinucleotide synthesis with Kang A relative to the same condition with Rif, normalized by the results with no antibiotic. Kang A has a strong inhibitory effect with GTP as the 5′-initiating nucleotide (blue bars), a weaker effect with GDP (red bars), and no inhibitory effect with GMP (green bars). The error bars denote standard error of four measurements