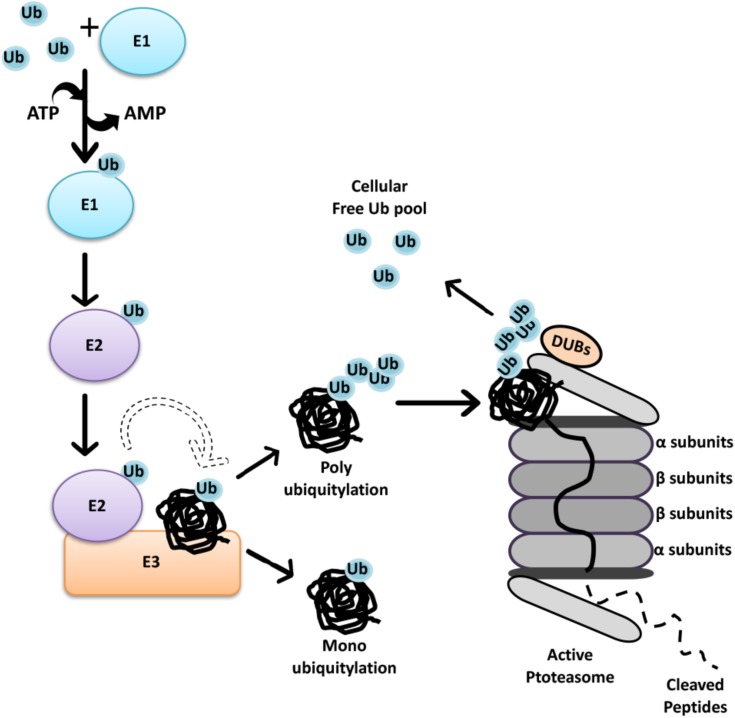
FIGURE 1.
The Ubiquitin-Proteasome System. Initially through C-terminal glycine, ubiquitin is attached to a cysteine residue of an activating enzyme, E1, in an ATP-dependent manner. The active ubiquitin is then associated with a cysteine residue of an ubiquitin conjugating enzyme, E2. Finally, specificity of ubiquitin transfer is ensured by E3 ubiquitin ligase family of proteins that bind to selected protein subsets (Hershko and Ciechanover, 1998). In the case of RING finger E3 ligases, the transfer of ubiquitin is direct from E2-ubiquitin to the substrate, even if the presence of E3 is required for substrate selection. At present, 2 genes are known to encode E1 isoforms, at least 40 genes encode E2’s, and over 600 E3 ubiquitin ligases were defined in the human genome (Pickart and Eddins, 2004; Clague et al., 2015). Each E1 isoform reveals a distinct preference for different E2 enzymes, while association of E2 and E3 depend on cellular context, generating extensive combinatorial complexity.