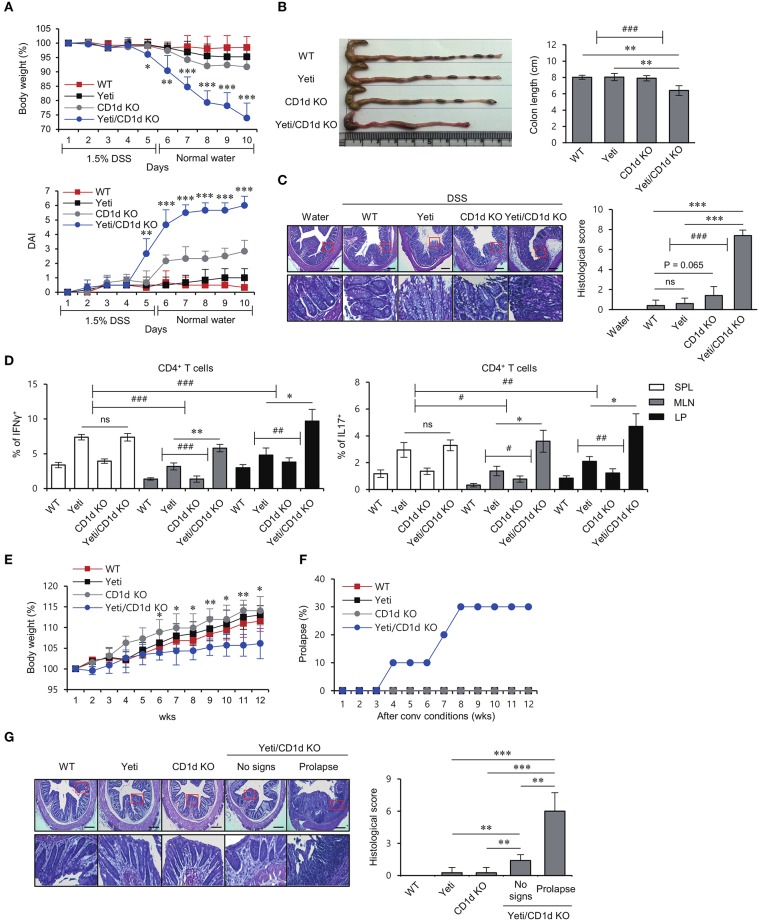
Figure 2.
Lack of iNKT cells accelerates intestinal inflammation in Yeti mice. Daily body weight changes, DAI score (A) and colon length (B) of WT, Yeti, CD1d KO, and Yeti/CD1d KO mice were evaluated after 1.5% DSS treatment. Data are representative of three independent experiments with similar results. (C) On day 10, distal colons from each group were sectioned and stained with H&E. (D) Intracellular IFNγ and IL17 production were assessed in splenic, MLN, and LP CD4+ T cells from these mice by flow cytometry on day 10. The mean values ± SD (n = 5 per group in the experiment; Student's t-test; *P < 0.05, **P < 0.01, ***P < 0.001) are shown. Two-way ANOVA (Yeti × iNKT and genotype × tissue) showed an interaction between these two factors (#P < 0.05, ##P < 0.01, ###P < 0.001). Daily body weight changes (E) and prolapse rate (F) of WT, Yeti, CD1d KO, and Yeti/CD1d KO mice housed for 12 weeks under conventional conditions. (n = 7 for WT, Yeti, and CD1d KO mice; n = 10 for Yeti/CD1d KO mice; Student's t-test; *P < 0.05, **P < 0.01). (G) Left, distal colons from these mice were sectioned and stained with H&E at week 12. Right, histologic damages were scored from H&E-stained sections at week 12. (n = 5 for WT, Yeti, and CD1d KO mice; n = 5 for Yeti/CD1d KO mice (no signs); n = 3 for Yeti/CD1d KO (prolapse); Student's t-test; **P < 0.01, ***P < 0.001).