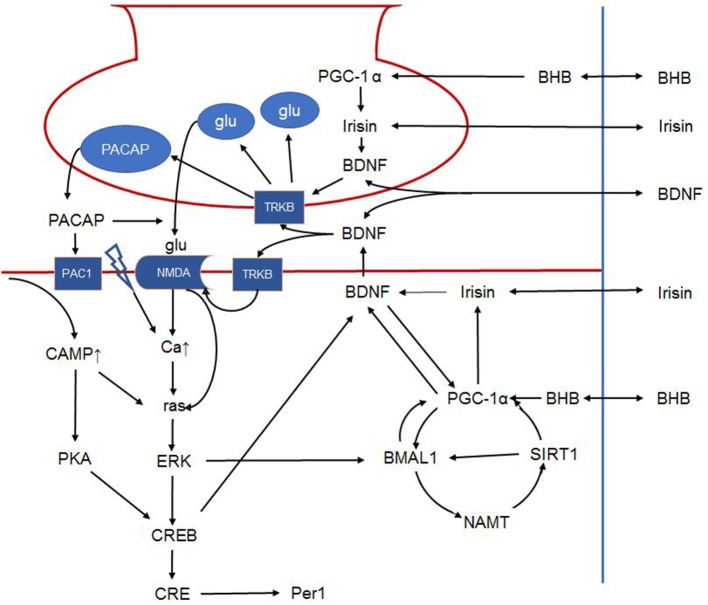
Figure 3.
Schematic outline of the intracellular signalization in the retinohypothalamic tract. Presynaptically, BDNF can increase glutamate and PACAP content of synaptic vesicles and facilitates their release. Postsynaptically, BDNF phosphorylates the NMDA receptor, increasing its number and the opening probability of the channel. BDNF furthermore enhances PACAP release. PAC1 receptor activation activates CREB via the cAMP-protein kinase A pathway. NMDA receptor activation leads to ras activation directly and indirectly by increasing intracellular calcium. Ras in turn activates ERK that on one hand activates CREB, and on the other phosphorylates BMAL1. In addition, BMAL1 is regulated by the NAMPT-SIRT1, PGC-1α cycle. Peripheral signals may further influence these complex interactions. For example, BHB induces PGC-1α that in turn induces irisin expression. Irisin's influence on BDNF expression and release is hypothetical in the SCN, however it was described in other brain areas. Hence, in the present article, we propose that peripheral signals (BHB, irisin etc.) liberated in response to non-photic environmental signals may modulate the permissive effect of BDNF. The link between irisin and BDNF in the SCN is hypothetical. BDNF: brain-derived neurotrophic factor; PACAP: pituitary adenylate cyclase-activating polypeptide; NMDA, N-methyl-D-aspartic acid; PAC1, pituitary adenylate cyclase-activating polypeptide type 1 receptor; CREB, cAMP response element-binding protein; cAMP, cyclic adenosine monophosphate; ERK, extracellular signal–related kinase; BMAL1, brain and muscle ARNT-like protein 1; NAMPT, nicotinamide phosphoribosyltransferase; SIRT1, sirtuin 1; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; BHB, β-hydroxybutyrate; SCN, suprachiasmatic nucleus.