Abstract
Fasudil, a Rho kinase (ROCK) inhibitor, effectively inhibits disease severity in a mouse model of Alzheimer's disease (AD). However, given its significant limitations, including a relatively narrow safety window and poor oral bioavailability, Fasudil is not suitable for long-term use. Thus, screening for ROCK inhibitor(s) that are more efficient, safer, can be used orally and suitable for long-term use in the treatment of neurodegenerative disorders is required. The main purpose of the present study is to explore whether FSD-C10, a novel ROCK inhibitor, has therapeutic potential in amyloid precursor protein/presenilin-1 transgenic (APP/PS1 Tg) mice, and to determine possible mechanisms of its action. The results showed that FSD-C10 effectively improved learning and memory impairment, accompanied by reduced expression of amyloid-β 1–42 (Aβ1–42), Tau protein phosphorylation (P-tau) and β-site APP-cleaving enzyme in the hippocampus and cortex area of brain. In addition, FSD-C10 administration boosted the expression of synapse-associated proteins, such as postynaptic density protein 95, synaptophsin, α-amino 3-hydroxy-5-methyl-4-isoxa-zolep-propionate receptor and neurotrophic factors, e,g., brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Taken together, our results demonstrate that FSD-C10 has therapeutic potential in the AD mouse model, possibly through inhibiting the formation of Aβ1–42 and P-tau, and promoting the generation of synapse-associated proteins and neurotrophic factors.
Keywords: Alzheimer's disease, APP/PS1transgenic mice, Rho kinase, FSD-C10
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder that occurs mainly in old age; it is characterized by deposits of amyloid-β (Aβ) plaques and neurofibrillary tangles, and neuronal loss (1), and its prevalence is rapidly increasing. It is likely that AD has multiple etiologies, although its precise cause remains unknown (2). Aβ and tau proteins constitute a prime neurotoxic component of senile plaques in the brain of AD patients, thus contributing to learning and memory impairment due to synaptic dysfunction and neuronal degeneration (3). However, to date most therapeutic interventions aimed at modifying a single pathological factor (e.g., cholinergic dysfunction, or Aβ aberrant processing) have failed because they target only limited pathogenic factors of AD (4). Inflammation, mitochondrial dysfunction, and oxidative stress are considered the most prominent concomitant pathological events (5–7), being potential targets of therapeutic intervention. Recently, the ER-associated degradation (ERAD) pathway has also been drawing widespread attention as control of protein-folding intermediaries in AD (8,9).
Inflammation has been proposed as a main factor in the pathogenesis of AD, including microglial activation, reactive astrocytes and inflammatory molecules (2,10–12). However, it has been noted that microglial activation exhibits both beneficial and detrimental effects depending on the stage of microglia (13). The conversion of microglia from detrimental (M1) to beneficial (M2) phenotype may contribute to an anti-inflammatory microenvironment in the brain (14). Similar to microglia, astrocytes also contribute to neuroinflammation in AD by releasing inflammatory cytokines and other toxic molecules (15). The ubiquitin-proteasome system and autophagy mechanisms are impaired due to the toxic effects of Aβ and oxidative stress damage, leading to the accumulation of oxidized/unfolded proteins that may contribute to neuronal loss (16). In fact, non-steroidal anti-inflammatory drugs (NSAIDS) initially garnered enthusiasm from pre-clinical and epidemiologic studies as agents for reducing the risk of AD (17), but anti-inflammatory treatment failed to produce beneficial effects in patients with severe cognitive impairment and dementia (18).
It has been reported that Rho activity, which is thought to contribute to AD pathogenesis (19), was elevated in the brain of AD model mice (20). Pharmacologic inhibition of Rho kinase (ROCK) induced protein degradation by autophagy in mammalian cells (21), and suppressed Aβ production in an AD mouse model (22), highlighting ROCK as a therapeutic target to combat Aβ production in AD. Fasudil, a selective ROCK inhibitor, increased dendrite branching and stabilized dendrite arbors in CA1 pyramidal neurons of APP/PS1 mice (23) by preventing neurodegeneration and stimulating neuroregeneration in various neurological disorders (19). Our previous study also confirmed that Fasudil treatment ameliorated memory deficits in APP/PS1 transgenic mice, accompanied by a decrease in Aβ deposits, p-Tau and BACE levels, an increase in PSD-95, and inhibition of the TLRS-NF-κB-MyD88 inflammatory cytokine axis (24).
Although previous studies have demonstrated certain beneficial effects of Fasudil intervention in the AD model (24), several lines of evidence suggest that there are some limitations in the clinical use of Fasudil, including its suitability only for short-course treatment, low oral bioavailability, a narrow safety window and blood pressure fluctuation. Thus, novel ROCK inhibitor(s) that are more efficient, safer, oral and suitable for long-term use for the treatment of neurodegenerative disorders are required. In the present study, we explored the therapeutic effect and systemic response of a novel ROCK inhibitor, FSD-C10, and possible mechanisms of its action in the treatment of a mouse model of AD.
Materials and methods
Animals and treatment
Experiments were performed on male APP/PS1 double transgenic mice (APPswe/PS1dE9, 8-month-old), purchased from Shanghai Research Center. Age-matched wild-type (WT) mice were used as controls. All animals were housed in a room maintained at 25°C with a 12-h light/dark cycle. The mice were given free access to food and water except during the behavioral test. The experiment was carried out in compliance with the Guidelines for Animal Care and Use of China, and approved by the Animal Ethics Committee of Shanxi Datong University, Datong, China (Ethical Approval no. 1601). Every effort was made to minimize suffering of the animals.
The experimental design was carried out in two stages: Validation of the AD model and intervention of the AD model. For validation of the AD model, mice were divided into two groups: Age-matched wild-type (n=8) and APP/PS1 transgenic mice (n=8). Mice were sacrificed at 8 months of age. Behavioral and pathological changes were measured before and after execution. For the intervention of FSD-C10, mice were divided into two groups: Normal saline (NS)-control mice (n=8) and FSD-C10-treated mice (n=8). Mice were treated with FSD-C10 (25 mg/kg/day every other day) or NS (0.9% NaCl) for 2 months by intraperitoneal (i.p.) injection. The concentration of FSD-C10 used in this study was adopted based on our preliminary experiments.
Mouse spatial learning and memory test
Spatial learning and memory of mice were assessed using the Morris water maze (MWM) test. The MWM is a 90 cm high, 50 cm diameter circular pool, containing a submerged escape platform (5.0×5.0 cm), 2.0 cm below the water surface. The pool was filled with water containing an edible white pigment that made the water opaque, and the water temperature was maintained at 23–25°C. In each trial, the time required to escape onto the hidden platform was recorded as escape latency. Mice were allowed a maximum of 60 sec to reach the platform, and if they failed to do so, they were guided to the platform. After training was completed, cognitive function was measured for 5 days, after which the platform was removed for spatial exploration in order to determine the memory capacity of the mouse as to the platform space position. All behavioral parameters of mice were tracked, recorded and analyzed using SMART 3.0 (Panlab, Barcelona, Spain).
Histopathology and immunohistochemistry
At the end of the final behavioral test, mice were anesthetized and transcardially perfused with NS and 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were sliced (10 µm) and analyzed by immunohistochemistry. Briefly, slides were blocked with 1% BSA (Sigma) for 1 h, and permeated with 0.3% Triton X-100 in 1% BSA/PBS for 30 min at RT. Sections were incubated with anti-Aβ1–42 (1:1,000; EMD Millipore, Billerica, MA, USA), anti-p-Tau (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-BACE (1:1,00; Cell Signaling Technology, Inc.), anti-synaptophin (1:1,000), anti-AMPAR-1 (1:1,000), anti-AMPAR-2 (1:1,000; all from Abcam, Cambridge, MA, USA), anti-BDNF (1:1,000; Promega Corporation, Madison, WI, USA), and anti-GDNF (1:1,000; Promega Corporation) at 4°C overnight. After washing, the slices were incubated with secondary antibodies at RT for 2 h. The nucleus was stained by Hoechst 33342 (1 µg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Control sections were run following identical protocols, but the primary antibodies were omitted. Results were visualized under fluorescence microscopy by IMAGE-PRO PLUS software (Media Cybernetics, Inc., Rockville, MD, USA). Quantification analysis of positive cells was performed on three sections per animal, and four mice per group were analyzed in a blinded fashion.
Western blot analysis
Proteins of mouse brain were extracted as previously described (17), Protein concentrations were determined by a bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology, Haimen, China). Protein samples were separated by SDS-PAGE and transferred onto PVDF membranes. After nonspecific binding was blocked with 5% nonfat dry milk, membranes were incubated at 4°C overnight with primary antibodies (Aβ1–42, p-Tau, BACE, PSD-95, AMPAR-1, AMPAR-2, BDNF, GDNF and β-actin). The next day, the membranes were washed three to four times with 0.1% Tween-20/TBST (pH 7.6) and incubated with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG secondary antibodies for 2 h at 37°C. To compare protein loading, antibodies directed against GAPDH or β-actin were used and relative optical density was measured with Image Lab Software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Graph Pad Prism 5 (Cabit Information Technology Co., Ltd., Shanghai, China) was used for statistical analysis. Results are presented as mean ± SEM. An unpaired, two-tailed Student's t-test was used for comparisons of means between two groups. In all tests, P<0.05 was considered to indicate a statistically significant difference.
Results
Behavioral and pathological changes in APP/PS1 mice at 8 months of age
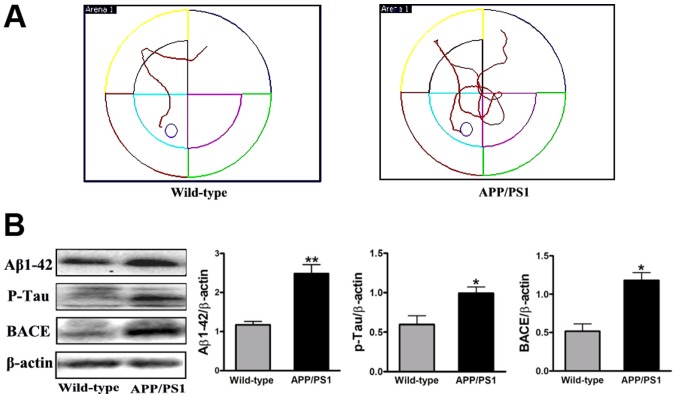
To observe the effect of FSD-C10 in APP/PS1 transgenic mice, we first tested the behavioral and pathological changes at the initiation time of drug intervention as a baseline. Memory and learning ability was measured by MWM and the expression of Aβ1–42, p-Tau and BACE protein was determined by western blot. As shown in Fig. 1, APP/PS1 transgenic mice exhibited obvious space learning and memory impairment at 8 months of age, as compared with the WT group. Simultaneously, the levels of Aβ1–42, p-Tau and BACE expression were more elevated in APP/PS1 transgenic mice than in the WT group (P<0.01, P<0.05 and P<0.05, respectively; Fig. 1B). Together, APP/PS1 mice showed the typical behavior dysfunction and typical pathologic abnormality of AD, thus confirming the AD model in these transgenic mice.
Figure 1.
AD transgenic mouse model. At 8 months of age, learning and memory ability was measured by the Morris Water Maze. (A) Compared with the WT group, APP/PS1 transgenic mice exhibited obvious space learning and memory impairment. (B) At the same time, expression of Aβ1–42, p-Tau and BACE protein was increased as measured by western blot. The results suggest that APP/PS1 mice showed typical behavior dysfunction and and typical pathologic abnormality of AD. Results are expressed as the fold change relative to β-actin as the loading control. Quantitative results are shown as mean ± SEM of 4 mice each group. *P<0.05 and **P<0.01 vs. Wild-type. AD, Alzheimer's disease.
FSD-C10 improves learning and memory abilities of the APP/PS1 Tg mice
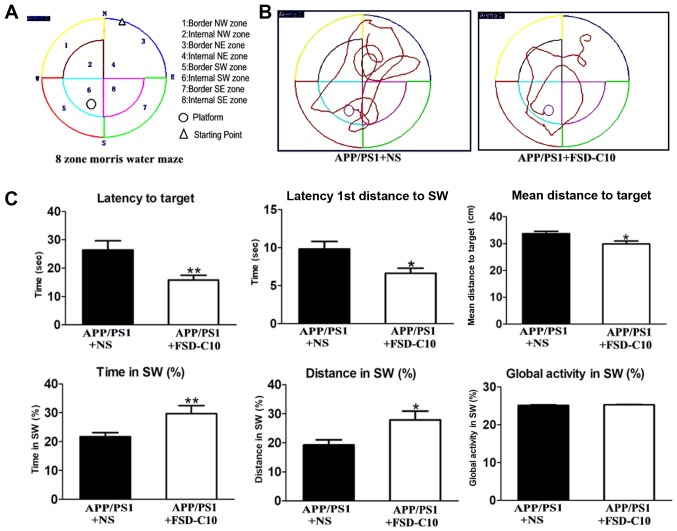
As shown in Fig. 1, APP/PS1 transgenic mice had obvious space learning and memory impairment at 8 months of age compared with the WT group. To observe whether FSD-C10 can ameliorate the deficit in learning and memory ability, MWM measurement was used in APP/PS1 mice after 2 months of treatment (Fig. 2A and B). As shown in Fig. 2C, FSD-C10 intervention significantly reduced the time and distance for Latency to Target (P<0.01), Latency 1st Entrance to SW (P<0.05), and mean distances to Target (P<0.05) of these AD mice.
Figure 2.
FSD-C10 improves behavioral deficits of APP/PS1 Tg mice. Cognitive ability was analyzed in a Morris water maze test. The Morris water maze test began two months after NS/FSD-C10 administration. (A) 8-zone Morris water maze schematic diagram, (B) typical diagram of two groups, and (C) Latency to Target, Latency 1st Entrance to SW, Mean Distances to Target, Time in SW (%), Distance in SW and Global Activity (%). Results are shown as mean ± SEM of 8 mice each group. *P<0.05 and **P<0.01 vs. APP/PS1+NS.
When the platform was moved, a consolidation of spatial memory was detected. The results showed that the movement of FSD-C10-treated mice mainly located on the position of the target platform quadrant, while NS-controlled mice mainly moved around the location of the target platform quadrant (Fig. 2A and B). The intervention of FSD-C10 increased Time in SW (P<0.01; Fig. 2C) and Distance in SW (P<0.05; Fig. 2C) as compared with the NS control group. However, there were no differences in global activity between two groups (P>0.05; Fig. 2C).
FSD-C10 attenuates Aβ burden, Tau phosphorylation and BACE expression
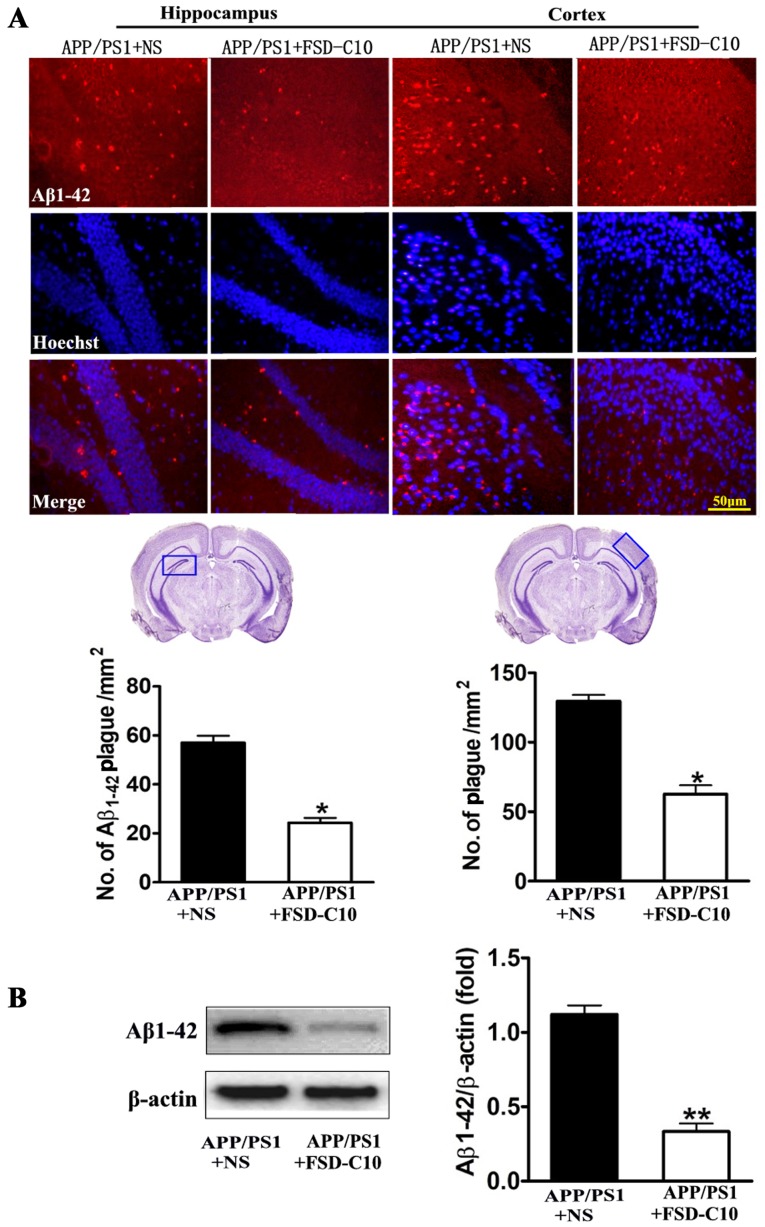
We evaluated the pathological changes in APP/PS1 transgenic mice, including Aβ plaques, Tau phosphorylation and BACE expression by immunohistochemistry and western blot. As shown in Fig. 3A, the numbers of Aβ1–42-positive cells in the hippocampus and cortex of brain were lower in FSD-C10-treated mice than in NS-treated control mice. Similarly, FSD-C10 intervention suppressed Aβ1–42 protein level in brain when compared with the NS-treated control group (P<0.01; Fig. 3B).
Figure 3.
FSD-C10 attenuates Aβ1–42 burden in hippocampus and cortex in APP/PS1 Tg mice. Aβ1–42 was measured by immunohistochemistry and western blot on two months after FSD-C10 administration. (A) Aβ1–42 expression was measured in hippocampus and cortex by immunohistochemistry and (B) in brain tissue by western blot. Quantitative results are shown as mean ± SEM of 4 mice each group, and one representative of three independent experiments. *P<0.05 and **P<0.01 vs. APP/PS1+NS.
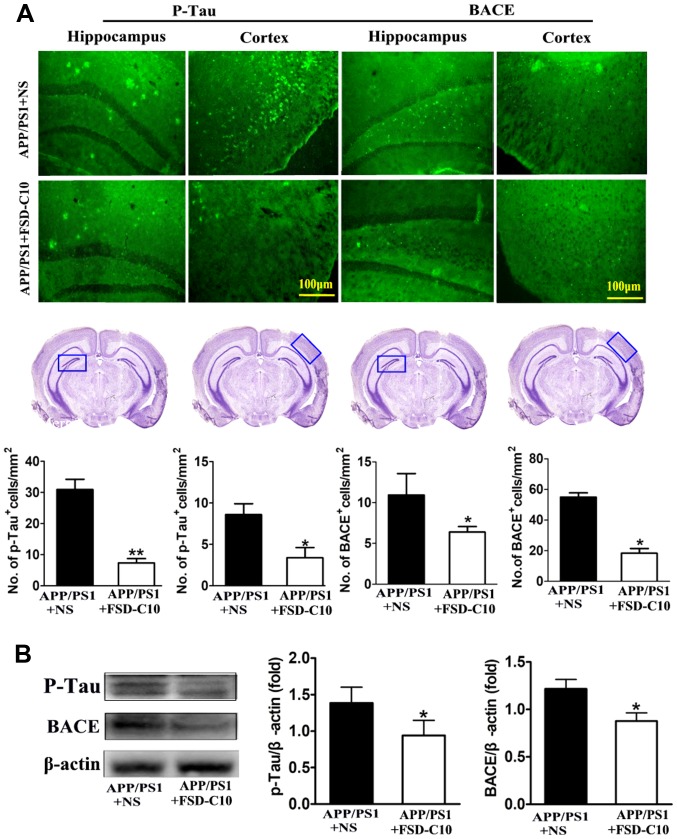
We further explored the levels of p-tau and BACE proteins in the hippocampus and cortex with immunohistochemistry and western blot. The results showed that the numbers of p-Tau and BACE positive cells were significantly lower in FSD-C10-treated group than in the NS-treated control group (P<0.05 and P<0.01 for p-Tau; P<0.05 and P<0.05 for BACE; Fig. 4A). Similarly, FSD-C10 treatment inhibited the expression of p-Tau and BACE protein in brain when compared with the NS-treated control mice (P<0.05; Fig. 4B). These results suggest that FSD-C10 treatment effectively alleviated pathological changes in AD mice.
Figure 4.
FSD-C10 reduces Tau phosphorylation and BACE expression in hippocampus and cortex in APP/PS1 Tg mice. p-Tau and BACE were measured by immunohistochemistry and western blot two months after FSD-C10 administration. (A) The numbers of p-Tau and BACE positive cells in hippocampus and cortex were measured by immunohistochemistry and (B) their protein expression was measured in brain by western blot. Quantitative results are shown as mean ± SEM of 4 mice each group, and one representative of three independent experiments. *P<0.05 and **P<0.01 vs. APP/PS1+NS.
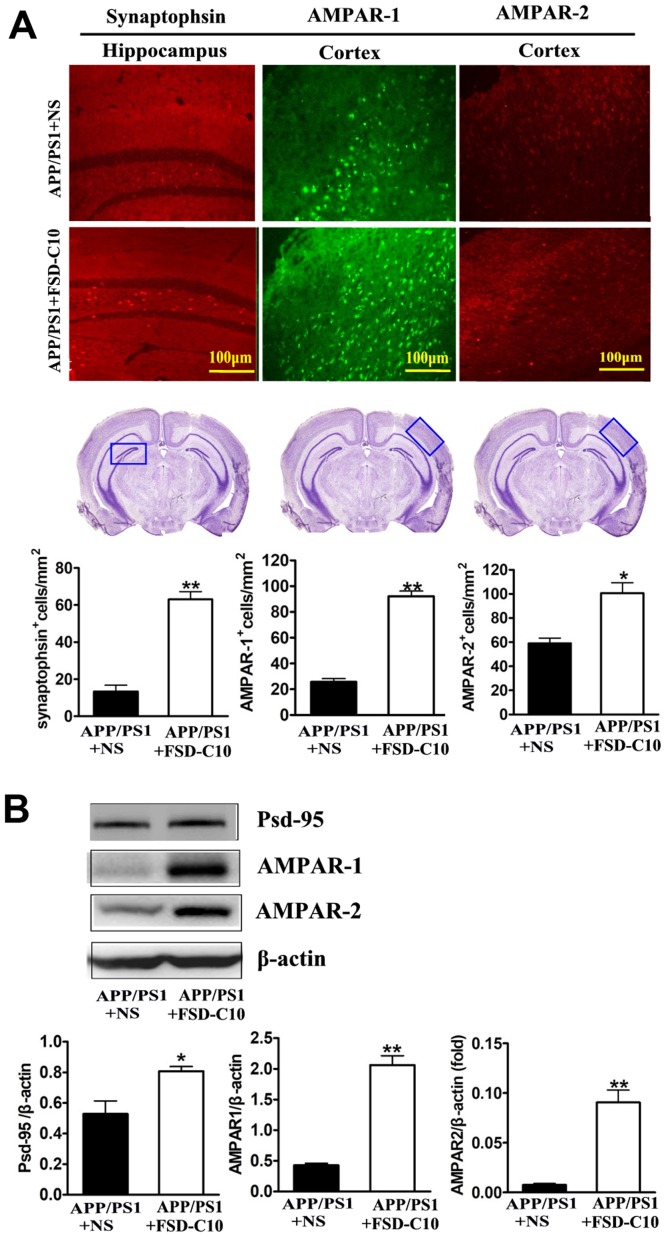
FSD-C10 induces the expression of synapse-associated proteins
It has become clear that cognitive dysfunction more strongly correlates with synapse loss in AD than with counts of plaques, tangles, and neuronal loss (25,26). Our results showed that FSD-C10 treatment up-regulated synaptophsin expression in the hippocampus (P<0.01; Fig. 5A) as well as AMPAR-1 and AMPAR-2 expression in the cortex (P<0.01 and P<0.05, respectively; Fig. 5A) as compared with NS-treated control mice. Data from western blot also confirmed that FSD-C10 treatment up-regulated the expression of AMPAR-1 and AMPAR-2 protein in brain (both P<0.01; Fig. 5B).
Figure 5.
FSD-C10 induces the expression of synaptophsin, AMPAR-1, AMPAR-2 and PSD-95 protein in hippocampus or cortex in APP/PS1 Tg mice. Synaptophsin, AMPAR-1, AMPAR-2 and PSD-95 were measured by immunohistochemistry and western blot two months after FSD-C10 administration. (A) The numbers of synaptophsin, AMPAR-1 and AMPAR-2 positive cells in hippocampus or cortex were measured by immunohistochemistry; (B) their protein in brain was measured by western blot. Quantitative results are shown as mean ± SEM of 4 mice each group, and one representative of three independent experiments. *P<0.05 and **P<0.01 vs. APP/PS1+NS.
PSD-95 is a synaptic protein that regulates synaptic distribution, synaptic stability and certain types of memory (27). As shown in Fig. 5B, FSD-C10 treatment elevated the level of PSD-95 expression in brain as compared with the NS-treated control group (P<0.05; Fig. 5B). These results indicate that the improvement in learning and memory impairment mediated by FSD-C10 may be related to the up-regulation of these synapse-associated proteins in both hippocampus and cortex.
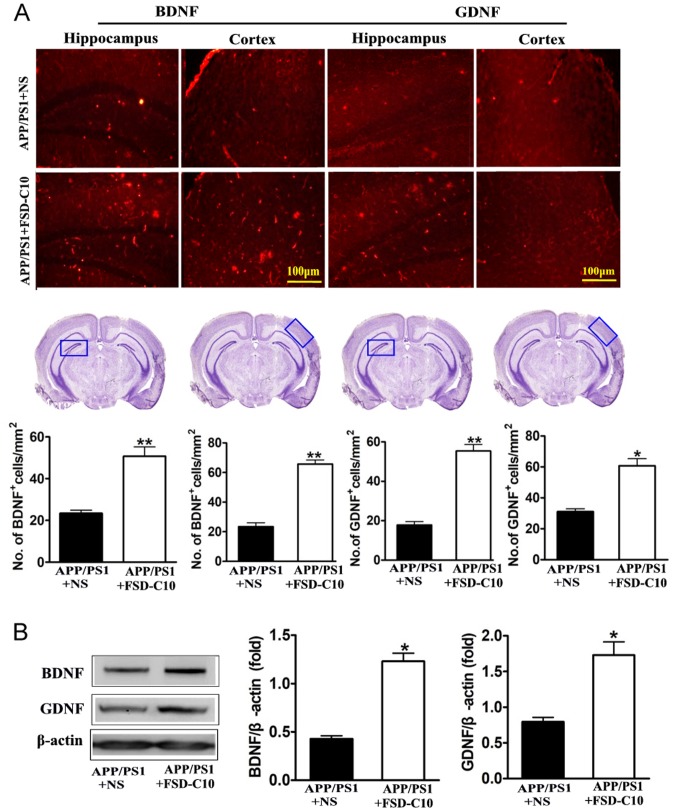
FSD-C10 increases the expression of neurotrophic factors
Neurotrophic factors are well known to be important for the survival, differentiation, growth and regeneration of neurons (28), as well as for synaptic transmission and plasticity (29). In this research, we explored the role of FSD-C10 in production of neurotrophic factors. Immunohistochemical analysis revealed that FSD-C10 intervention increased the numbers of both BDNF and GDNF positive cells in the hippocampus and cortex of brain compared with the NS-treated control group (P<0.01 and P<0.05 respectively; Fig. 6A). Expression of BDNF and GDNF protein was also higher in FSD-C10-treated mice than in the NS-treated control group measured by western blot (both P<0.05; Fig. 6B). FSD-C10 thus has a potentially neuroprotective effect through the production of neurotrophic factors.
Figure 6.
FSD-C10 promotes the expression of neurotrophic factors in hippocampus or cortex in APP/PS1 Tg mice. The expression of BDNF and GDNF protein was measured by immunohistochemistry and western blot two months after FSD-C10 administration. (A) The numbers of BDNF and GDNF positive cells in hippocampus and cortex by immunohistochemistry; and (B) their protein expression in brain by western blot. Quantitative results are shown as mean ± SEM of 4 mice each group, and one representative of three independent experiments. *P<0.05 and **P<0.01 vs. APP/PS1+NS.
Discussion
AD is an age-related and chronic neurodegenerative disorder presenting as progressive cognitive decline. Although the exact pathogenesis is not yet clear, multiple etiologic pathways have been considered (2). The major histopathological hallmarks of AD include Aβ plaques and neurofibrillary tangles (NFTs) formed by hyperphosphorylated Tau protein (30), and the neurite atrophy and synaptic loss induced by Aβ are considered to be the major cause of gradual cognitive detetrioration in AD (31). Neurotoxicity of Aβ becomes apparent via induction of oxidative stress, neuronal excitotoxicity, neuroinflammation and apoptosis (32,33). One of the most promising strategies for treatment of AD is to directly target Aβ by decreasing the production and clearing aggregation of Aβ (34). In addition to Aβ accumulation, tau hyperphosphorylation is also an important pathological hallmark of AD (35). Abnormal phosphorylation of tau protein promotes the loss of microtubule stabilizing ability and may contribute to neurite degeneration as well as NFT formation (36). Current drugs for AD treatment, such as acetylcholinesterase inhibitor and NMDA antagonist, show limited benefits in most AD patients (37). There is thus an urgent need for novel therapeutic strategies that can halt the disease process and are suitable for long-term clinical management. In our previous studies, a comparative study of Fasudil and FSD-C10 has been reported, including cell viability, cell death, neurite outgrowth and dendritic formation, vasodilation insensitivity and animal mortality (38). In this study, we demonstrate that treatment with FSD-C10, as a new ROCK inhibitor, was able to reverse cognitive impairment, possibly through decreasing Aβ accumulation and Tau phosphorylation in the cortex and hippocampus of APP/PS1 transgenic mice.
A series of studies have demonstrated that ROCK was elevated in the brain of AD patients compared to controls (39,40), and inhibition of ROCK activity decreased Aβ levels by enhancing APP protein degradation (41). Abnormal activation of ROCK has also been found in AD experimental models, and may be involved in the occurrence and development of diseases (41). ROCK activation increased Aβ production, CRMP-2 phosphorylation and hindered tubulin assembly, leading to the inhibition of synapses (20,42). Inhibition of ROCK reduced the production of Aβ (41) and increased the synaptic density and length of hippocampal pyramidal neurons (43). Fasudil has been proved to protect against nerve degeneration induced by Aβ, and to improve spatial memory and learning ability in AD rats (44). Our present study provides further evidence that inhibiting ROCK activity can improve cognitive deficits in APP/PS1 transgenic mice.
In clinical practice, Fasudil has several limitations, including a relatively narrow safety window, poor oral bioavailability, and unsuitability for long-term use. Researchers are looking for novel ROCK inhibitors that are more efficient and safer, can be taken orally and over a long period of time for the treatment of neurodegenerative disorders. We have in previous studies found that FSD-C10, as a novel ROCK inhibitor, has the same therapeutic effect, but is safer than Fasudil (38). In EAE, FSD-C10 ameliorated the clinical severity of disease, accompanied by improvement in demyelination and the inhibition of inflammatory cells in the CNS of EAE mice, clearly showing a therapeutic effect (45).
Glutamate, the major excitatory neurotransmitter in the CNS, is involved in synaptic transmission, neuronal growth and differentiation, synaptic plasticity and learning and memory (46). When a neuron is depolarized, glutamate is released into the synaptic cleft where it binds glutamate receptors (47). Glutamate receptors, such as NMDA receptor and AMPA receptor, are involved in rapid excitatory synaptic transmission and the release of neurotransmitters, which is closely related with learning and memory (48). In the postsynaptic membrane, an AMPAR insert can induce and promote learning and memory behavior. Aβ-induced decline in AMPAR number and synaptic function through endocytosis is a plausible mechanism for the cognitive impairment that occurs in the very early stages of AD (49). Further study found that lack of AMPAR can cause dendritic spine reduction and loss of NMPAR (50), both of which are related to cognitive impairment. In our study, increased expression of AMPAR, synaptophsin and PSD-95 protein after FSD-C10 administration could be an important mechanism for the improvement in cognitive function in transgenic APP/PS1 mice. PSD-95 organizes synaptic proteins to mediate the functional and structural plasticity of the excitatory synapse and to maintain synaptic homeostasis (51). The stabilization of a new synaptic protrusion is associated with activity-driven PSD proteinaceous network formation. In this proteinaceous network, PSD-95 is believed to play a role in synapse maturation, given that it is particularly vulnerable to the toxic effects of Aβ (28). Synaptophysin is the major integral membrane protein of presynaptic vesicles required for vesicle formation and exocytosis (52). Taken together, FSD-C10 may maintain the normal function of the synapse, possibly through promoting the expression of these synaptic related proteins.
Neurotrophic factors play an essential role in the survival of neurons affected by degenerative processes (53,54). Increased levels of BDNF are associated with improved learning and memory, and a reduction in BDNF leads to age-related memory deficits (55). The potential of GDNF against age-related cognitive deterioration has not been fully explored. It was reported that serum GDNF levels were significantly reduced in AD patients (56,57), and expression of GDNF transgene in astrocytes improved cognitive deficits in aged rats (58). Thus, increased BDNF, GDNF, AMPAR, PSD-95, or synaptophysin levels may reflect increased synaptic density, activity, and vesicles, revealing improved functioning of synapses. Inducing expression of these molecules could therefore be a mechanism underlying improved learning and memory abilities of the APP/PS1 transgenic mice after FSD-C10 treatment. The limitations of this study may include that further mechanisms underlying the inhibitory effect on Aβ after blocking ROCK activity in AD remain unknown, and the oral effect of this reagent in AD mice needs be tested. These questions will be further explored in the near future.
Acknowledgements
The authors would like to thank Ms. Katherine Regan (Department of Neurology, Thomas Jefferson University) for editorial assistance.
Funding
The present study was supported by grants from The National Natural Science Foundation of China (grant nos. 81272163, 81471412 and 81371414), Shanxi University of Traditional Chinese Medicine (grant no. 2011PY-1), research projects supported by Datong Municipal Science and Technology Bureau (grant no. 2017136) and Shanxi Datong University (grant no. 2016K10), and an open project of The State Key Laboratory of Molecular Developmental Biology of China (grant no. 2018-MDB-KF-07 to YQY).
Availability of data and materials
The analysed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QFG and JZY designed the study, performed the mouse behavioral tests, participated in the statistical analysis and revised the manuscripts. CGM conceived the study, participated in its design and coordination, and helped to draft the manuscript. BGX designed and performed all analyses, and drafted and revised the manuscript. GXZ participated in the study design and revised the manuscript. YHL performed the immunoassays. CYL performed the western blot analysis. HW and LF performed the statistical analysis and the immunohistochemistry. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experiment was carried out in compliance with the Guidelines for Animal Care and Use of China, and approved by the Animal Ethics Committee of Shanxi Datong University, Datong, China (Ethical Approval no. 1601).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 2.Khalil Bou R, Khoury E, Koussa S. Linking multiple pathogenic pathways in Alzheimer's disease. World J Psychiatry. 2016;6:208–214. doi: 10.5498/wjp.v6.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrer I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol. 2012;97:38–51. doi: 10.1016/j.pneurobio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer'sdisease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 5.Jiang T, Sun Q, Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's diseaseand Alzheimer's disease. Prog Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Onyango IG, Dennis J, Khan SM. Mitochondrial dysfunction in alzheimer's disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7:201–214. doi: 10.14336/AD.2015.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 8.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran-Aniotz C, Cornejo VH, Hetz C. Targeting endoplasmic reticulum acetylation to restore proteostasis in Alzheimer's disease. Brain. 2016;139:650–652. doi: 10.1093/brain/awv401. [DOI] [PubMed] [Google Scholar]
- 10.Hoeijimakers L, Heinen Y, van Dam AM, Lucassen PJ, Korasi A. Microglial priming and Alzheimer's disease: A possible Role for (Early) immune challenges and epigenetics? Front Hum Neurosci. 2016;10:398. doi: 10.3389/fnhum.2016.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen I, Singhrao SK. Inflammation involvement in alzheimer's disease. J Alzheimer's Dis. 2016;54:45–53. doi: 10.3233/JAD-160197. [DOI] [PubMed] [Google Scholar]
- 12.Torika N, Asraf K, Roasso E, Danon A, Fleisher-Berkovich S. Angiotensin converting enzyme inhibitors ameliorate braininflammation associated with microglial activation: Possible implication for Alzheimer's disease. J Neueoimmune Pharmacol. 2016;11:774–785. doi: 10.1007/s11481-016-9703-8. [DOI] [PubMed] [Google Scholar]
- 13.Wallker DG, Lue LF. Immune phenotypes of microglial in humane neurodegenerativedisease: Challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Gripe A, Axt D, Remus A, Remus A, et al. NLRP3 is actived in Alzheimer's disease and contributes to pathology in APP/PS1mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trammtola A, Di Domenico F, Barone E, Perluigi M, Butterfield DA. It is all about (U)biquitin: role of altered ubiquitin-proteasome system and UCHLI in Alzheimer's Disease. Oxid Med Cell Longev 2756068. 2016 doi: 10.1155/2016/2756068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deardorff WJ, Grossberg GT. Targeting neuroinflammation in Alzheimer's disease: Evidence for NASIDs and novel therapeutics. Expert Rev Neurother. 2017;17:17–32. doi: 10.1080/14737175.2016.1200972. [DOI] [PubMed] [Google Scholar]
- 18.Miguel-Álvarez M, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Garatachea N, Lucia A. Non-steroidal anti-inflammmatory drugs as a treatment for Alzheimer's disease: A systematic review and meta-analysis of treatment effect. Drugs Aging. 2015;32:139–147. doi: 10.1007/s40266-015-0239-z. [DOI] [PubMed] [Google Scholar]
- 19.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurogical disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 20.Petraos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH. The beta-amyloid protein of Alzheimer's disease increase neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain. 2008;131:90–108. doi: 10.1093/brain/awm260. [DOI] [PubMed] [Google Scholar]
- 21.Bauer PO, Wong HK, Oyama F, Goswami A, Okuno M, Kino Y, Miyazaki H, Nukina N. Inhbition of Rho kinases enhances the degradation of mutant huntingtin. J Boiol Chem. 2009;284:13153–13164. doi: 10.1074/jbc.M809229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herskowitz JH, Feng Y, Mattheyses AL, Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC, Thambisetty M, Seyfried NT, et al. Pharmacologic inhibition of ROCK2 suppresses amyloid-β production in an Alzheimer's disease mouse model. J Neurosci. 2013;33:19086–19098. doi: 10.1523/JNEUROSCI.2508-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couch BA, DeMarco GJ, Gourley SL, Koleske AJ. Increased dendrite branching in AbetaPP/PS1 mice and elongation of dendrite arbors by fasudil administration. J Alzheimers Dis. 2010;20:1003–1008. doi: 10.3233/JAD-2010-091114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu JZ, Li YH, Liu CY, Wang Q, Gu QF, Wang HQ, Zhang GX, Xiao BG, Ma CG. Multitarget therapeutic effect of fasudil in APP/PS1transgenic mice. CNS Neurol Disord Drug Targets. 2017;16:199–209. doi: 10.2174/1871527315666160711104719. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 27.Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prvulovic D, Hampel H. Amyloid β (Aβ) and phospho-tau (p-tau) as diagnostic biomarkers in Alzheimer's disease. Clin Chem Lab Med. 2011;49:367–374. doi: 10.1515/CCLM.2011.087. [DOI] [PubMed] [Google Scholar]
- 31.Tohda C, Ichimura M, Bai Y, Tanaka K, Zhu S, Komatsu K. Inhibitory effects of Eleutherococcus senticosus extracts on amyloid beta(25–35)-induced neuritic atrophy and synaptic loss. J Pharmacol Sci. 2008;107:329–339. doi: 10.1254/jphs.08046FP. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZH, Yu LJ, Hui XC, Wu ZZ, Yin KL, Yang H, Xu Y. Hydroxy-safflor yellow A attenuates Aβ1–42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway. Brain Res. 2014;1563:72–80. doi: 10.1016/j.brainres.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Morroni F, Sita G, Tarozzi A, Rimondini R, Hrelia P. Early effects of Aβ1–42 oligomers injection in mice: Involvement of PI3K/Akt/GSK3 and MAPK/ERK1/2 pathways. Behav Brain Res. 2016;314:106–115. doi: 10.1016/j.bbr.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 35.Mateo I, Vázquez-Higuera JL, Sánchez-Juan P, Rodríguez-Rodríguez E, Infante J, García-Gorostiaga I, Berciano J, Combarros O. Epistasis between tau phosphorylation regulating genes (CDK5R1 and GSK-3beta) and Alzheimer's disease risk. Acta Neurol Scand. 2009;120:130–133. doi: 10.1111/j.1600-0404.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 36.Goedert M. Neurofibrillary pathology of Alzheimer's disease and other tauopathies. Prog Brain Res. 1998;117:287–306. doi: 10.1016/S0079-6123(08)64022-4. [DOI] [PubMed] [Google Scholar]
- 37.Cummings J, Aisen PS, DuBois B, Frölich L, Jack CR, Jr, Jones RW, Morris JC, Raskin J, Dowsett SA, Scheltens P. Drug development in Alzheimer's disease: The path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin YL, Yu JZ, Yang XW, Liu CY, Li YH, Feng L, Chai Z, Yang WF, Wang Q, Jiang WJ, et al. FSD-C10: A more promising novel ROCK inhibitor than Fasudil for treatment of CNS autoimmunity. Biosci Rep. 2015;35 doi: 10.1042/BSR20150032. pii: e00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salminen A, Suuronen T, Kaarniranta K. ROCK, PAK, and Toll of synapses in Alzheimer's disease. Biochem Biophys Res Commun. 2008;371:587–590. doi: 10.1016/j.bbrc.2008.04.148. [DOI] [PubMed] [Google Scholar]
- 40.Bobo-Jiménez V, Delgado-Esteban M, Angibaud J, Sánchez-Morán I, de la Fuente A, Yajeya J, Nägerl UV, Castillo J, Bolaños JP, Almeida A. APC/CCdh1-Rock2 pathway controls dendritic integrity and memory. Proc Natl Acad Sci USA. 2017;114:4513–4518. doi: 10.1073/pnas.1616024114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson BW, Gentry EG, Rush T, Troncoso JC, Thambisetty M, Montine TJ, Herskowitz JH. Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer's disease and ROCK1 depletion reduces amyloid-β levels in brain. J Neurochem. 2016;138:525–531. doi: 10.1111/jnc.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arimura N, Ménager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, Fukata Y, Amano M, Goshima Y, Inagaki M, et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Biol. 2005;25:9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanger SA, Mattheyses AL, Gentry EG, Herskowitz JH. ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cell Logist. 2016;5:e1133266. doi: 10.1080/21592799.2015.1133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song Y, Chen X, Wang LY, Gao W, Zhu MJ. Rho kinase inhibitor fasudil protects against β-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci Ther. 2013;19:603–610. doi: 10.1111/cns.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li YH, Yu JZ, Liu CY, Zhang H, Zhang HF, Yang WF, Li JL, Feng QJ, Feng L, Zhang GX, et al. Intranasal delivery of FSD-C10, a novel Rho kinase inhibitor, exhibits therapeutic potential in experimental autoimmune encephalomyelitis. Immunology. 2014;143:219–229. doi: 10.1111/imm.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ß peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38:6–23. doi: 10.1503/jpn.110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masoudian N, Riazi GH, Afrasiabi A, Modaresi SM, Dadras A, Rafiei S, Yazdankhah M, Lyaghi A, Jarah M, Ahmadian S, Seidkhani H. Variations of glutamate concentration within synaptic cleft in the presence of electromagnetic fields: An artificial neural networks study. Neurochem Res. 2015;40:629–642. doi: 10.1007/s11064-014-1509-6. [DOI] [PubMed] [Google Scholar]
- 48.Anggono V, Tsai LH, Götz J. Glutamate receptors in Alzheimer's disease: Mechanisms and therapies. Neural Plast. 2016;2016:8256196. doi: 10.1155/2016/8256196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong Z, Han H, Li H, Bai Y, Wang W, Tu M, Peng Y, Zhou L, He W, Wu X, et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J Clin Invest. 2015;125:234–247. doi: 10.1172/JCI77888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keith D, El-Husseini A. Excitation control: Balancing PSD-95 function at the synapse. Front Mol Neurosci. 2008;1:4. doi: 10.3389/neuro.02.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- 53.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 54.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: Current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/S0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 55.Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: Relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/S0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 56.Straten G, Eschweiler GW, Maetzler W, Laske C, Leyhe T. Glial cell-line derived neurotrophic factor (GDNF) concentrations in cerebrospinal fluid and serum of patients with early Alzheimer's disease and normal controls. J Alzheimers Dis. 2009;18:331–337. doi: 10.3233/JAD-2009-1146. [DOI] [PubMed] [Google Scholar]
- 57.Forlenza OV, Miranda AS, Guimar I, Talib LL, Diniz BS, Gattaz WF, Teixeira AL. Decreased neurotrophic support is associated with cognitive decline in non-demented subjects. J Alzheimers Dis. 2015;46:423–429. doi: 10.3233/JAD-150172. [DOI] [PubMed] [Google Scholar]
- 58.Pertusa M, García-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, Cristòfol R, Sarkis C, Sanfeliu C. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analysed data sets generated during the present study are available from the corresponding author on reasonable request.