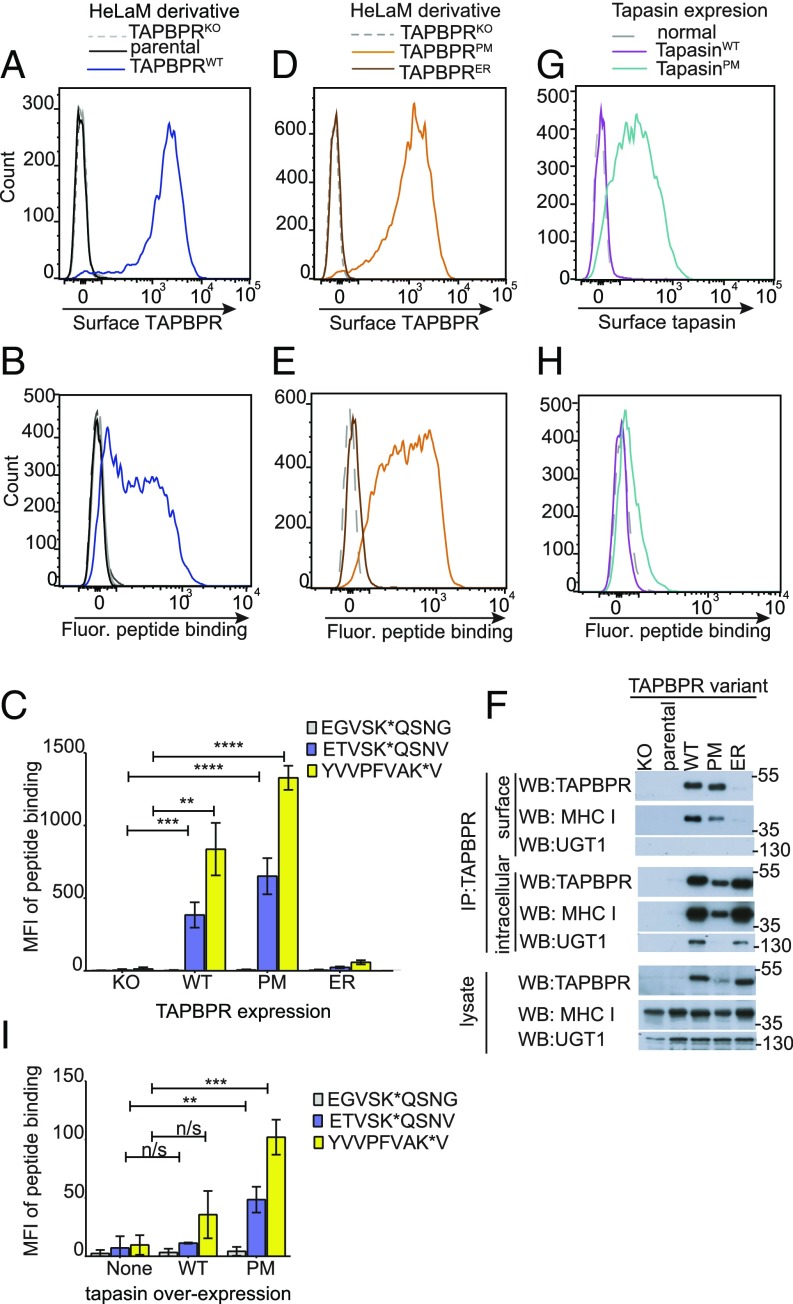
Fig. 1.
Surface-expressed TAPBPR enhances exogenous peptide association onto MHC I. Surface expression of (A and D) TAPBPR, detected using the mAb PeTe-4 or (G) tapasin, detected using Pasta1 on IFN-γ–treated (A) HeLaM cells and HeLaM-TAPBPRKO ± TAPBPRWT transduction, (D) HeLaM-TAPBPRKO ± TAPBPRPM or TAPBPRER transduction, or (G) HeLaM-TAPBPRKO ± tapasinWT or tapasinPM transduction. Staining with an isotype control (solid black line) is included in A. Note: HeLaM-TAPBPRKOTAPBPRPM cells with low transduction levels were selected to generate cells with similar surface expression as TAPBPRWT. (B, E, and H) Histograms show the typical peptide binding observed when the cells were incubated with the HLA-A*68:02-specific fluorescent peptide ETVSK*QSNV at 10 nM for 15 min at 37 °C. (C and I) Bar charts summarizing the level of exogenous fluorescent peptide binding when cells were incubated with 10 nM EGVSK*QSNG, ETVSK*QSNV, or YVVPFVAK*V for 15 min at 37 °C. Bars show mean fluorescence intensity (MFI) ± SD from three independent experiments. n/s not significant, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 using unpaired two-tailed t test. (F) Immunoprecipitation of the cell surface pool of TAPBPR, by staining intact cells with PeTe-4 before lysis and addition of protein-A Sepharose, and the remaining intracellular TAPBPR pool from cells postsurface TAPBPR preclear, followed by Western blotting for TAPBPR, MHC I (using HC10), or UGT1 on immunoprecipitates and lysates as indicated. Data shown is representative of three independent experiments. For comparison, a classic coimmunoprecipitation from these cells is also provided (SI Appendix, Fig. S1D).