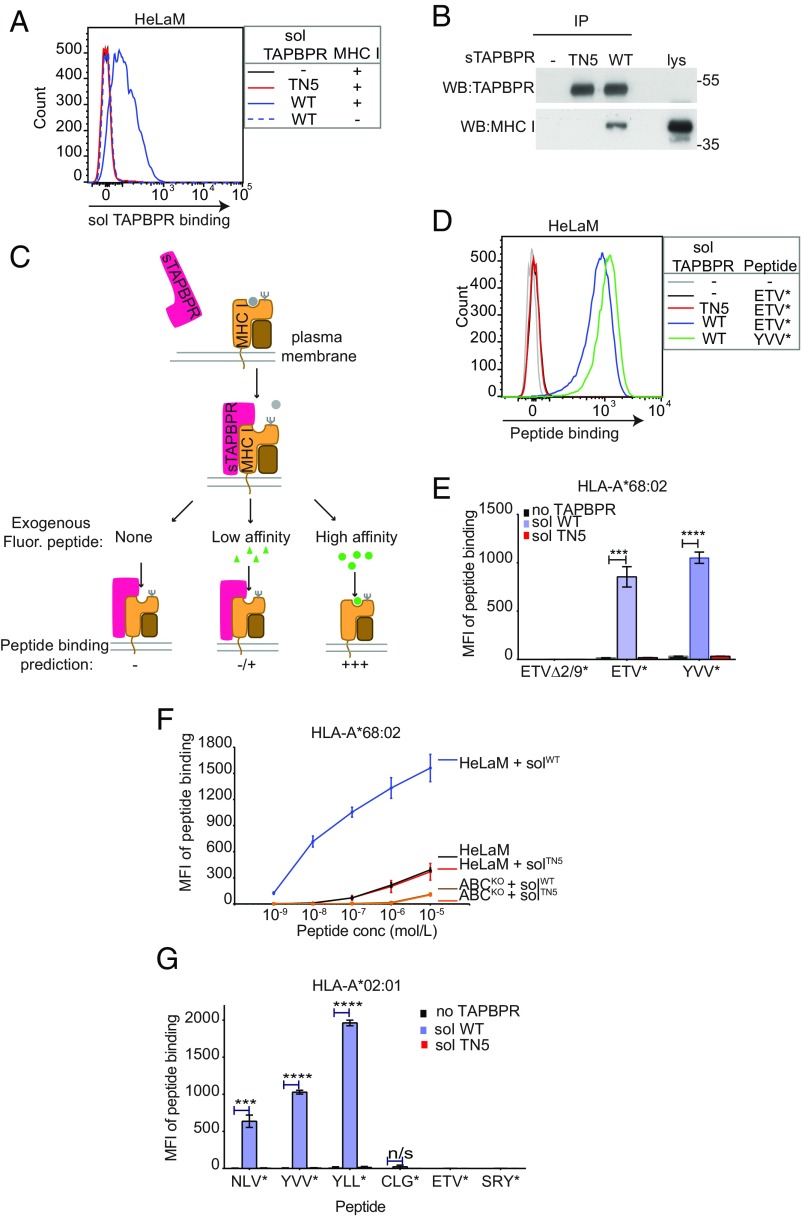
Fig. 3.
Soluble TAPBPR enhances exogenous peptide association onto surface MHC I. (A) IFN-γ–treated HeLaM cells were incubated ±100 nM soluble TAPBPRWT or TAPBPRTN5 for 15 min at 37 °C, followed by detection of surface-bound TAPBPR using PeTe-4. Soluble TAPBPRWT binding to HeLaM-HLA-ABCKO cells (MHC−) is included as a control. (B) TAPBPR pulldowns on lysates from IFN-γ–treated HeLaM-TAPBPRKO cells incubated ±soluble TAPBPRTN5 or TAPBPRWT demonstrate that TAPBPRWT binds to MHC I. Data are representative of three independent experiments. (C) Schematic representation of experimental workflow used to measure peptide exchange by soluble TAPBPR. (D and E) IFN-γ–treated HeLaM cells were incubated ±100 nM soluble TAPBPRWT or TAPBPRTN5 for 15 min at 37 °C, followed by incubation ±10 nM ETVSK*QSNV (ETV*), YVVPFVAK*V (YVV*), or EGVSK*QSNG (ETVΔ2/9) for 15 min at 37 °C. In D, histograms show the typical binding observed for ETVSK*QSNV and YVVPFVAK*V and E shows the MFI of fluorescent peptide binding ±SD from three independent experiments. (F) Dose–response curves ±SD from three independent experiments of ETVSK*QSNV binding to IFN-γ–treated HeLaM and HeLaM-HLA-ABCKO cells treated ±100 nM TAPBPR with increasing concentrations of peptide for 15 min at 37 °C. (G) Bar graph show the MFI of fluorescent peptide binding to IFN-γ–treated HeLaM-HLA-ABCKO cells reconstituted with HLA-A*02:01 ± SD from two independent experiments with duplicates. Cells were incubated in the absence or presence of 1 µM soluble TAPBPRWT or TAPBPRTN5 for 15 min, followed by incubation with 10 nM NLVPK*VATV (NLV*), YVVPFVAK*V (YVV*), YLLEK*LWRL (YLL*), CLGGK*LTMV (CLG*), ETVSK*QSNV (ETV*), or SRYWK*IRTR (SRY*) for 60 min. ***P ≤ 0.001, ****P ≤ 0.0001, n/s not significant, using unpaired two-tailed t test.