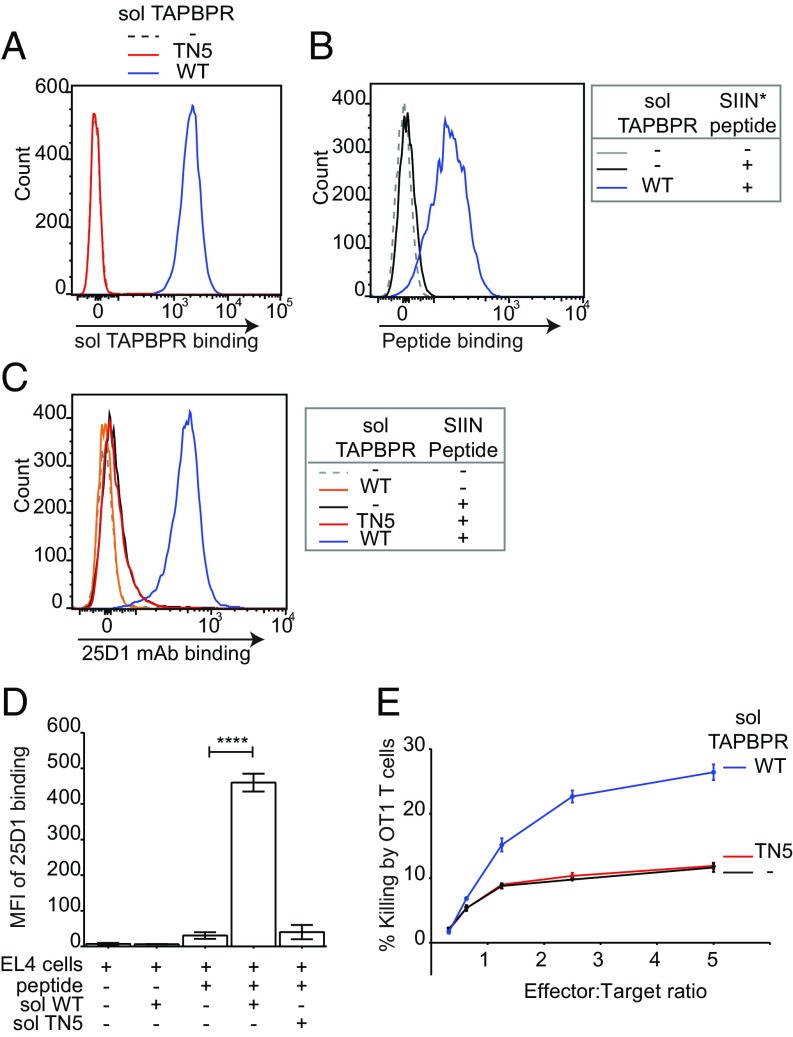
Fig. 5.
Soluble TAPBPR enhances T cell killing of tumor cells. EL4 cells were incubated ±1 µM soluble TAPBPRWT or TAPBPRTN5 for 15 min at 37 °C, followed by (A) detection of surface bound TAPBPR using PeTe-4, (B) incubation ±1 nM SIINFEK*L for 30 min at 37 °C, or (C) incubation ±1 nM nonlabeled SIINFEKL peptide for 30 min, followed by staining with the 25-D1.16 mAb (recognizes SIINFEKL/H-2Kb complexes). Histograms are representative of three independent experiments. (D) Bar graphs show the MFI of 25-D1.16 ± SD from three independent experiments. (E) OT1 killing of EL4 cells treated ±1 µM soluble TAPBPRWT or TAPBPRTN5, followed by incubation with 1 nM SIINFEKL peptide. Error bars ± SEM from triplicate wells. Data are representative of three independent experiments. Note: surface expressed H-2Kb are relatively more peptide receptive compared with human MHC I molecules. At 10 nM SIINFEKL, some exogenous peptide binding was observed in the absence of soluble TAPBPRWT. Because OT1 T cells are highly efficient cytotoxic cells, killing 80–100% of targets after 1–4 h, we decreased the concentration of SIINFEKL used in these experiments to 1 nM to differentiate between TAPBPR-mediated and background peptide binding; otherwise, we would not observe an additive effect of soluble TAPBPR on target cell killing. ****P ≤ 0.0001 using unpaired two-tailed t test.