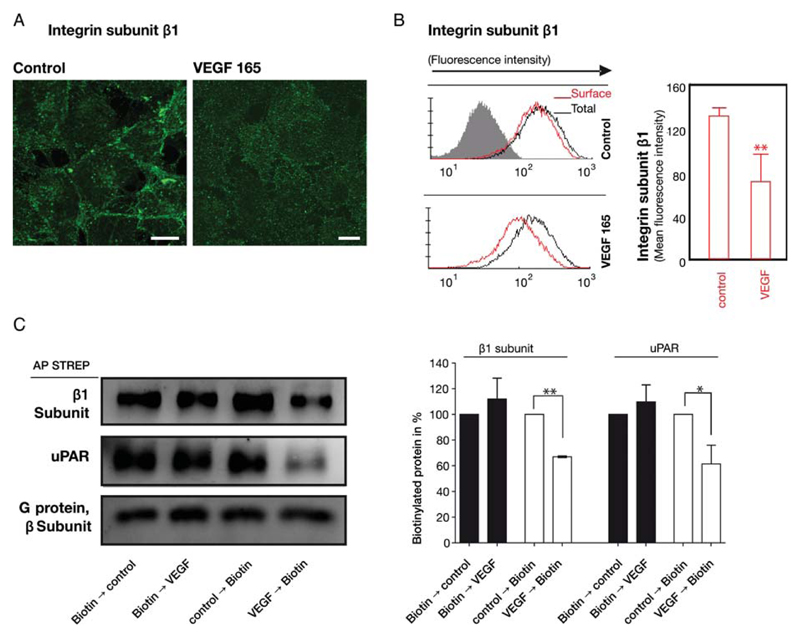
Figure 1.
VEGF-induced internalization of β1-integrins in endothelial cells. (A) VEGF165 stimulated redistribution of β1-integrins visualized by confocal microscopy: the integrin subunit β1 (green) was detected by indirect immunofluorescence in fixed, permeabilized human umbilical vein endothelial cells (HUVECs); scale bars 10 μm. (B) Internalization of β1-integrins was determined by flow cytofluorimetry of non-permeabilized (cell surface β1, red histogram) and permeabilized (total cellular β1 amount, black histogram) HUVECs incubated in the absence and presence of VEGF165 (50 ng/mL) for 15 min. The bar diagram summarizes the quantitative analysis (mean ± standard deviation) of cell surface β1-integrins calculated from the geometric mean fluorescence values (n = 3). (C) Cell surface proteins were biotinylated either before or after VEGF165 stimulation for 60 min and enriched by affinity precipitation on streptavidin beads from detergent extracts; biotinylated uPAR and β1-integrins was detected by immunoblotting and quantified by densitometry relative to the unstimulated control, which was the 100% reference value. Immunoblotting with an antiserum recognizing all G protein β-subunits was done as a loading control. Mean ± standard deviation (n = 3), **P < 0.01, t-test for paired data.