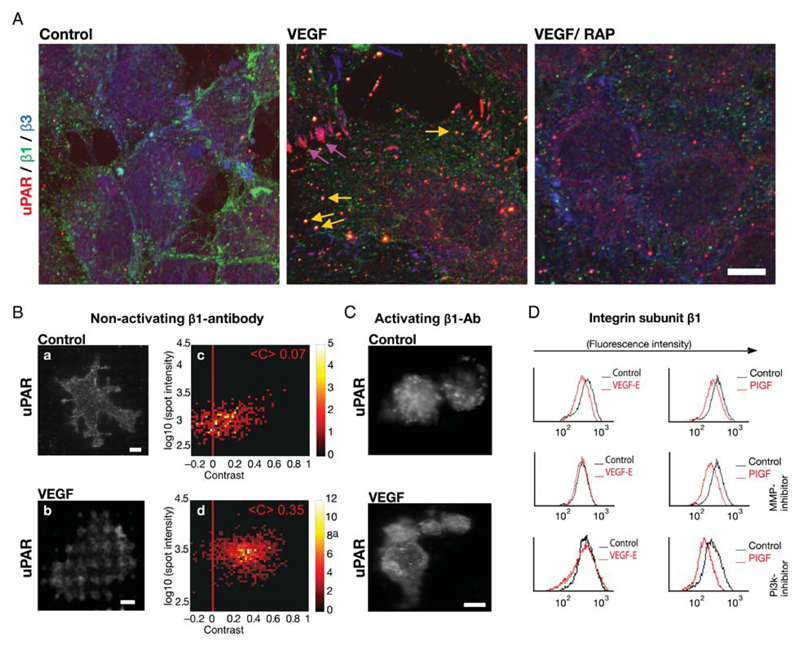
Figure 2.
VEGF-induced co-internalization of β1-integrins and uPAR. (A) Co-localization of uPAR with β1-integrins and with integrin-αvβ3 in intracellular vesicles and in focal adhesions, respectively, of VEGF165-stimulated HUVECs that were fixed and subjected to triple immunofluorescence staining for β3-integrins (green), uPAR (red), and β1-integrins (blue). Scale bar 10 μm. (B) Cell surface interaction between uPAR and β1-integrins assessed by micropatterning. Endothelial cells were grown on functionalized glass coverslips, with grids of BSA-Cy5 printed on them and interspaces coated with streptavidin and biotinylated non-activating monoclonal antibody against integrin subunit β1. After stimulation with VEGF165 (50 ng/mL) for 60 min, samples were fixed and immunostained with a pre-labelled uPAR monoclonal antibody. The VEGF-induced redistribution of uPAR was visualized by TIRF-microscopy. Scale bar 6 μm. Statistical analysis for the single spot fluorescence brightness F and contrast C of multiple cells (n = 64) is represented in a colour density plot with the mean contrast indicated. (C) HUVECs were grown and treated as in (B) on a surface patterned by immobilizing an activating antibody against β1-integrins, which precluded any appreciable VEGF-induced redistribution of uPAR. (D) HUVECs pre-treated with wortmannin (0.1 μM; PI3-kinase inhibitor) or MMP2/9 inhibitor (1 μM) were stimulated with either VEGF-E or PIGF. (50 ng/mL, each). The surface levels of β1-integrins were determined by flow cytometry as in Figure 1B.