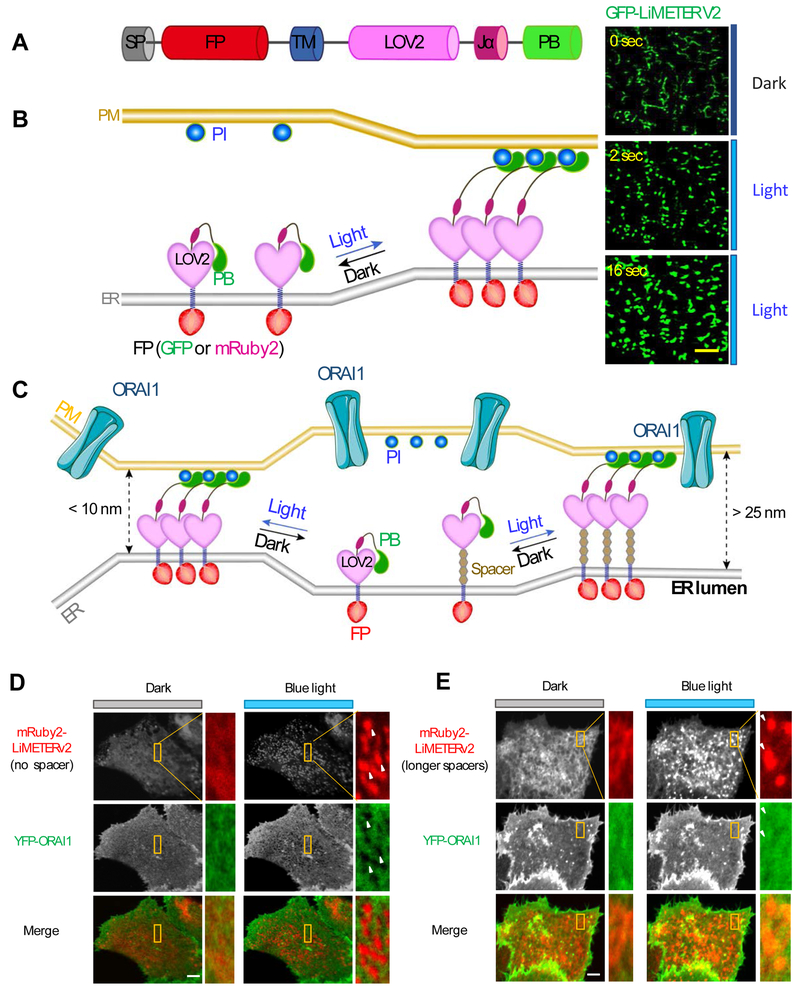
Figure 3 ∣. Mimicking reversible ER-PM MCS formation with LiMETER.
(A) LiMETER is engineered from STIM1 by replacing the luminal EF-SAM with a fluorescent protein (FP) and substituting the majority of its cytosolic domain with the LOV2 photosensitive module. The ER-targeting signal peptide (SP) and transmembrane domain (TM) were used to retain the synthetic protein to ER, whereas the C-terminal PB affords the tethering function through protein-phosphoinositide (PI) interaction. In the prototypical design, PB is derived from the Rit GTPase. In a recently improved version (also termed as OptoPBer or LiMETERv2), the PB is isolated from STIM1.
(B) Schematic representation of the working principle of LiMETER. In the dark, the Jα helix tightly docks to the core body of LOV2, thus imposing steric hindrance to the C-terminally fused PB domain to prevent its association with PM-resident Pis. Upon light illumination, the Jα helix unwinds and releases the constraints on PB, allowing itself to bind to the Pis to bridge the gap between ER and PM apposition. Shown on the right are typical images of GFP-tagged LiMETERv2 under a TIRF microscope following blue light irradiation.
(C) LiMETER can be engineered to manipulate the gap distance between ER-PM MCSs. Spacers with varying lengths can be modularly inserted between the TM and LOV2 domains to bridge a range of gap distances at MCSs. A gap distance of over 25 nm is required to allow efficient diffusion of ORAI1 proteins into MCS to evoke Ca2+ influx.
(D-E) Representative images of HeLa cells co-expressing YFP-ORAI (green) and mRuby-LiMETERv2 (red) with no spacer (D) or a long spacer (E). The yellow boxed regions were enlarged for a better visualization of the relative distribution of the two components in the same cell. YFP-ORAI1 was excluded from ER-PM MCSs (arrows) marked by mRuby-LiMETERv2 in the absence of the spacer (D). By contrast, YFP-ORAI1 can freely diffuse into ER-PM MCSs labeled by mRuby-LiMETERv2 with an appropriate spacer (E).