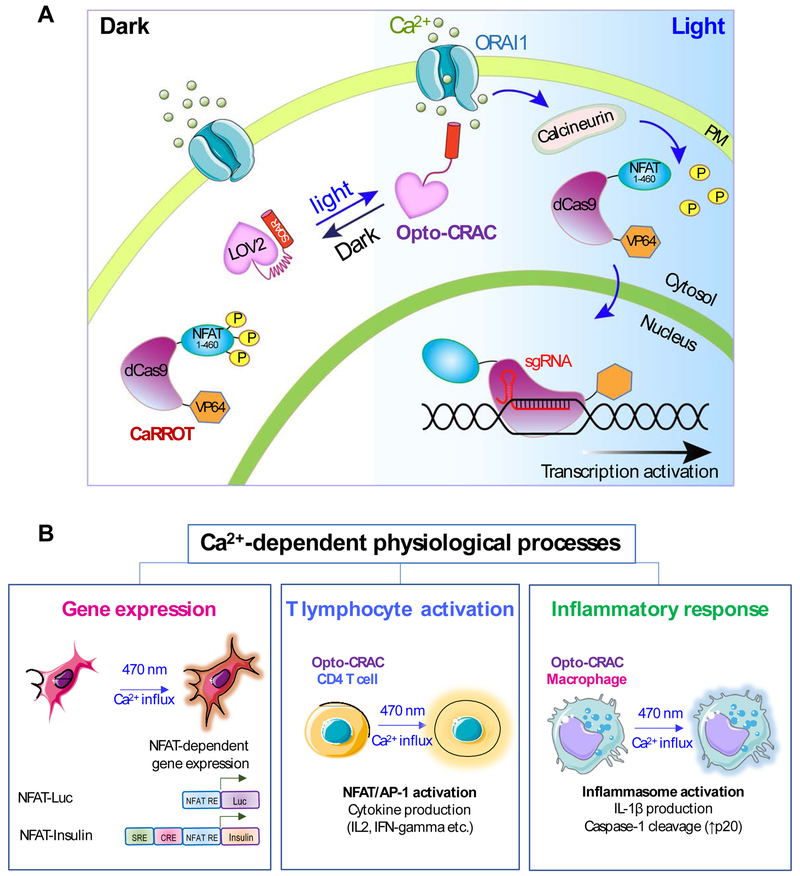
Figure 4 ∣. Examples illustrating potential applications of STIM1-derived optogenetic tools.
(A) Rewiring photoactivatable Ca2+ influx for light-inducible modulation of gene expression. Upon light stimulation, Opto-CRAC induces cytosolic Ca2+ influx to activate the Ca2+/CaM-dependent phosphatase, calcineurin, which in turn dephosphorylates the N-terminal fragment of NFAT (NFAT1-460) to drive the nuclear entry of a synthetic transcription regulatory device made of catalytically-dead Cas9 and the transcriptional coactivator VP64 (NFAT1-460-dCas9-VP64; designated as CaRROT for Ca2+-responsive transcriptional reprogramming tool). In the presence of small guide RNAs (sgRNAs), CaRROT can be precisely targeted to specific genomic loci to photo-tune the expression of endogenous genes to achieve tailored function. Likewise, other effector domains such as transcriptional repressors (e.g., KRAB) and epigenetic regulators (DNA or histone modifying enzymes) [95, 111, 112] can be installed to replace VP64, thereby enabling photoswitchable inhibition of gene expression and epigenetic remodeling.
(B) Opto-CRAC can be applied to photo-control Ca2+-dependent gene expression (left), cytokine production in T lymphocytes (middle), and inflammasome activation in macrophage (right). Optogenetic immunomodulation is likely to be of translational values for remote and personalized control of the immune system to fight invading pathogens and cancer with high precision.