ABSTRACT
Neutrophils sense and respond to diverse chemotactic cues through G-protein-coupled receptors (GPCRs). However, the precise trafficking dynamics of chemoattractant GPCRs during neutrophil activation and chemotaxis remain unclear. Here, by using small-molecule inhibitors and CRISPR-based knockouts, we establish that two primary chemoattractant GPCRs – formyl peptide receptor 1 (FPR1) and complement component 5a (C5a) receptor 1 (C5aR1) – internalize in a CDC42–actin-dependent manner. Through live-cell imaging, we demonstrate that, upon stimulation, FPR1 rapidly clusters and re-distributes along the plasma membrane to the trailing edge, where it internalizes and is directionally trafficked towards the front of migrating primary human neutrophils. In contrast to FPR1 and C5aR1, the leukotriene B4 (LTB4) receptor (BLT1, also known as LTB4R), which relays LTB4 signals in response to primary chemoattractants during neutrophil chemotaxis, fails to internalize upon physiological stimulation with LTB4, N-formyl-Met-Leu-Phe (fMLF) or C5a. Importantly, we report that blocking the LTB4–BLT1 axis or downstream myosin activation enhances the internalization of FPR1 and C5aR1, thus reducing downstream signaling and impairing chemotaxis to primary chemoattractants. The polarized trafficking of chemoattractant GPCRs and its regulation by the BLT1-mediated myosin activation therefore drives persistent chemotactic signaling in neutrophils.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: G-protein-coupled receptor, Endocytosis, Neutrophils, Leukotriene B4, Myosin, Chemotaxis
Summary: The leukotriene B4–BLT1 axis regulates the polarized trafficking of the chemoattractant receptors FPR1 and C5aR1 from the trailing edge of polarized neutrophils, to drive persistent chemotaxis.
INTRODUCTION
Neutrophils are part of the first line of defense during inflammation. They sense and rapidly respond to diverse inflammatory cues by acquiring polarity and migrating directionally to inflammation sites (Vargas et al., 2017; Hind et al., 2016). In response to activation by inflammatory cues, neutrophils secrete a variety of proteases, produce reactive radical species, phagocytose particles and form extracellular traps (neutrophil extracellular traps; NETs) (Thieblemont et al., 2016). Most of these functional responses are the consequence of signaling cascades activated in response to inflammatory cues or chemoattractants, which act largely through receptors belonging to the G-protein-coupled receptor (GPCR) superfamily (Artemenko et al., 2014).
Chemoattractant GPCRs regulate the functions of multiple leukocyte subtypes, including neutrophils. The formyl-peptide receptor 1 (FPR1), for example, is expressed by most innate leukocyte subtypes, and is activated upon binding of formylated peptides derived from bacteria during infections or from mitochondria upon necrosis resulting from cellular injury (Chen et al., 2017). FPR1 is among the most widely studied chemoattractant GPCR in the context of neutrophil activation and functional responses (He and Ye, 2017). Like other GPCRs, once activated, FPR1 is endocytosed and desensitized – a response characterized by the phosphorylation of its intracellular tail by a GPCR kinase (GRK) (Cotton and Claing, 2009). However, most studies pertaining to internalization and trafficking of chemoattractant receptors, such as FPR1 and complement component 5a (C5a) receptor 1 (C5aR1), have been limited to their expression and characterization in heterologous cell culture systems, such as fibroblasts (Vines et al., 2003), HEK 293 cells (Gilbert et al., 2001) or CHO cells (Suvorova et al., 2005). Although β-arrestin and GRK2, which are associated with the clathrin-mediated endocytic pathway, have been shown to mediate internalization of C5aR1 and FPR1 in RINm5F cells and neutrophil-like PLB-985 cells, respectively (Braun et al., 2003; Liu et al., 2012), evidence supporting clathrin-independent endocytosis of C5aR1 and FPR1 has also been established (Gilbert et al., 2001; Suvorova et al., 2005). Furthermore, little is known about the spatio-temporal dynamics of chemoattractant GPCRs in fast-migrating amoeboid-like cells, such as neutrophils.
Among chemoattractants, the formylated peptide N-formyl-Met-Leu-Phe (fMLF) and C5a are known ‘end-target’ chemoattractants that are stronger and can override chemotactic cues from ‘intermediate’ chemoattractants such as leukotriene B4 (LTB4) or CXCL8 (Foxman et al., 1997; Heit et al., 2002). Upon the addition of end-target or primary chemoattractants, such as fMLF, neutrophils produce and secrete the intermediate or secondary chemoattractant LTB4, which then acts on its cognate GPCR BLT1 (also known as LTB4R) to activate downstream signals (Subramanian et al., 2017). In fact, the LTB4–BLT1 axis acts as a signal-relay mechanism to amplify chemotactic responses to primary chemoattractants in neutrophils (Afonso et al., 2012; Pazos et al., 2015). In addition, upon fMLF stimulation neutrophils actively package LTB4 as well as the machinery required for LTB4 synthesis into multi-vesicular bodies that are secreted as exosomes to facilitate the formation of a secondary chemoattractant gradient (Majumdar et al., 2016). The released LTB4 acts in an autocrine and paracrine manner to enforce cell polarization and elicit a secondary gradient that extends the recruitment range of neutrophils to primary chemoattractants. Although LTB4 has been shown to mediate F-actin stability and myosin activation (Afonso et al., 2012), the precise contribution of BLT1 signaling to bolster directional migration in response to primary chemoattractants remains unclear.
In this study, by using specific small-molecule inhibitors, established endocytic markers and CRISPR-based knockouts, we identify the mode of internalization and the trafficking fate of the primary chemoattractant GPCRs FPR1 and C5aR1, as well as the relay receptor BLT1, in neutrophils. Furthermore, by using live-cell imaging, we establish the spatio-temporal dynamics of GPCR endocytosis and trafficking. Finally, we identify the specific contribution of BLT1 signaling in the regulation of internalization of primary chemoattractant GPCRs, and its impact on downstream signaling and neutrophil chemotaxis.
RESULTS
FPR1 internalization is independent of GRK2 and clathrin
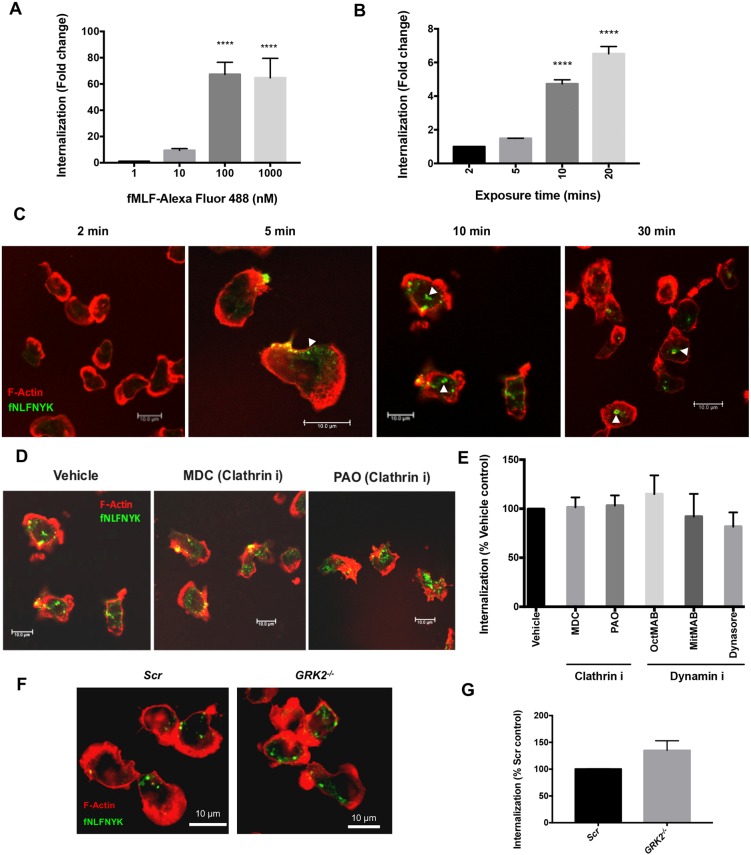
To investigate the mechanisms underlying FPR1 internalization, we used the FPR1 ligands fMLF conjugated to Alexa Fluor 488 (fMLF–Alexa-Fluor-488) and N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys conjugated to fluorescein (fNLFNYK–FITC) to directly follow ligand-bound receptors in primary human neutrophils (polymorphonucleated neutrophils; PMNs) and used the spot-detection method to quantify the extent of internalization (see Materials and Methods for details). Using a 10-fold difference in the dose range of fMLF–Alexa-Fluor-488, we observed that maximum internalization occurred when fMLF–Alexa-Fluor-488 was added at 100 nM (Fig. 1A). When added at a physiological concentration [2 nM fNLFNYK–FITC; the Kd for FPR1 is ∼3 nM (Gilbert et al., 2001)], we detected a time-dependent internalization (Fig. 1B), with strong membrane-bound ligand clusters appearing as early as 5 min post addition, by which time the cells were highly polarized, and a strong internalized FITC signal was detected from 10 min onwards (Fig. 1C; white arrowheads).
Fig. 1.
FPR1 endocytosis is independent of dynamin and GRK2. (A) Graph depicting the extent of internalization in PMNs treated with different doses (nM) of fMLF–Alexa-Fluor-488 for 10 min before fixation and staining for F-actin and nuclei. The extent of internalization is compared to 1 nM stimulation from n=3 independent experiments and represented as mean±s.e.m. ****P≤0.0001 (one-way ANOVA with Dunnett's multiple comparisons test). (B) Graph depicting the extent of internalization of fNLFNYK–FITC in PMNs treated with fNLFNYK–FITC (2 nM) for the indicated time points before fixation and staining for F-actin and nuclei. The extent of internalization is compared to that in a 2 min stimulation from n=3 independent experiments and represented as mean±s.e.m. ****P≤0.0001 (one-way with Dunnett's multiple comparisons test). (C) Representative confocal images of PMNs treated with fNLFNYK–FITC (green; 2 nM) for the indicated time points, then washed, fixed and co-stained with phalloidin (red). White arrowheads indicate internalizing or internalized fNLFNYK and the presence of F-actin around the fNLFNYK signals. Images are representative of three independent experiments. (D) Representative confocal images of PMNs pre-treated with vehicle [0.2% (v/v) DMSO], or endocytosis mode inhibitors (100 µM MDC and 500 nM PAO) for 30 min and treated with fNLFNYK–FITC (2 nM) for 10 min, and fixed and co-stained with phalloidin (red). Images are representative of three independent experiments. (E) Graph depicting the extent of internalization of fNLFNYK–FITC in PMNs pre-treated with vehicle [0.2% (v/v) DMSO] or endocytosis inhibitors (denoted with an ‘i’ after the protein targeted) shown in D and others (500 nM OctMAB, 2.5 µM MitMAB, 25 µM Dynasore and 50 µM MβCD) for 30 min and treated with fNLFNYK–FITC (2 nM) for 10 min. The extent of internalization is compared to that in vehicle control with n=3 independent experiments and represented as mean±s.e.m. No significant differences were noted between conditions (one-way ANOVA with Dunnett's multiple comparisons test). (F,G) Representative confocal sections of dPLBs targeted with Scr or GRK2 sgRNAs treated with fNLFNYK–FITC (10 nM; green) for 15 min, and fixed and co-stained with phalloidin (red). Images are representative of two independent experiments (F). The extent of fNLFNYK internalization is compared to Scr controls with n=4 independent experiments and represented as mean±s.e.m. No significant differences were noted between conditions (non-parametric paired Welch's two-tailed t-test) (G). Scale bars: 10 µm.
To verify the specificity of internalization of FPR1 ligands and the efficiency of the spot-detection assay, we took the following approaches. First, we imaged the potential internalization of the non-chemotactic ligand anti-α3 integrin antibody conjugated to Alexa Fluor 488 (α3 integrin antibody–Alexa Fluor 488). As PMNs do not express α3 integrin receptors on their surface (Rieu et al., 1993), this antibody represents a good way to control for potential non-specific fluid phase uptake/micropinocytosis of particles from the surrounding medium by polarized PMNs. To assess the internalization of the α3 integrin antibody, cells were co-stained with CellTracker and CellMask, which label the cytoplasm and the plasma membrane, respectively, before stimulation with fMLF. We found that upon stimulation of PMNs with fMLF, the α3 integrin antibody failed to internalize into the cytoplasm of PMNs (Fig. S1A). However, under similar conditions, fMLF–Alexa-Fluor-488 readily internalized into cytoplasmic vesicles (Fig. S1A; blue arrowheads). Second, we compared the extent of internalization of α3 integrin antibody–Alexa Fluor 488 with fMLF–Alexa-Fluor-488 through two different methods: (1) the total fluorescence intensity and (2) the spot-detection method. We found that irrespective of the method used, conjugated fMLF was internalized strongly (>30-fold higher) compared to α3 integrin antibody–Alexa Fluor 488 (Fig. S1B), despite using excess α3 integrin antibody–Alexa Fluor 488 (1 µg) compared to fMLF–Alexa-Fluor-488 (416 pg/ml).
Next, we tested whether the signals detected by the spot-detection method we employed were indeed intracellular. To achieve this, we performed experiments with fMLF–Alexa-Fluor-488 and used an anti-Alexa Fluor 488 antibody to quench membrane signals. To test whether the anti-Alexa Fluor 488 antibody was membrane impermeable and quenched membrane-bound Alexa Fluor 488 signals, we first added anti-β2 integrin antibody conjugated to Alexa Fluor 488 (β2 integrin antibody–Alexa Fluor 488) to PMNs and treated them with fMLF (10 nM) for 10 min, followed by fixation and treatment with or without the anti-Alexa Fluor 488 antibody. We observed a dense membrane staining along the rear of polarized PMNs as well as a pool of internalized β2 integrin signals (Fig. S1C; orange arrowheads indicate staining at the membrane). Importantly, treatment with the anti-Alexa Fluor 488 antibody completely abolished the membrane-bound signals of the β2 integrin antibody–Alexa Fluor 488, but the cells retained internalized β2 integrin signals (Fig. S1C). These findings establish that anti-Alexa Fluor 488 antibody quenched membrane-bound Alexa Fluor 488 signals. Next, we treated PMNs with 10 nM fMLF–Alexa-Fluor-488 for 10 min, fixed and treated with or without the anti-Alexa Fluor 488 antibody. Unlike the results with the β2 integrin antibody–Alexa Fluor 488, we failed to observe any membrane-bound fMLF–Alexa-Fluor-488 signals and no difference was observed in the extent of detection of the internalized pool of fMLF–Alexa-Fluor-488 signals upon treatment with or without the anti-Alexa Fluor 488 antibody (Fig. S1C,D). Therefore, our assay specifically detected internalized ligands and not plasma membrane-bound ligands. Furthermore, we analyzed the extent of internalization of fMLF–Alexa-Fluor-488 in the absence or presence of the membrane-impermeable anti-Alexa Fluor 488 antibody and compared the results using the total fluorescence intensity and spot-detection assays, respectively. Again, irrespective of the method used, we failed to detect any difference in the extent of fMLF–Alexa-Fluor-488 internalization between the anti-Alexa Fluor 488 antibody-treated and untreated samples (Fig. S1D). Taken together, these findings establish that the spot-detection method is as sensitive as the total fluorescence intensity measurements in identifying internalized FPR1 ligands.
To establish that FPR1 specifically binds and uptakes the conjugated ligand, we used the pluripotent hematopoietic cell line PLB-985 (PLB), which is amenable to genetic manipulation and can readily be induced to differentiate into neutrophil-like cells (Tucker et al., 1987), and generated FPR1−/− PLBs using the CRISPR technology. FPR1−/− PLBs were characterized by the presence of a frame-shift deletion next to the PAM site, which is detected by Cas9 to introduce double-stranded breaks in the target site, in the first 250 bps of the FPR1 coding region and not in the predicted off-target locus ANKK1 [Table S1; Fig. S1E, compare scramble (Scr) with FPR1 single-guide (sg)RNA]. Functionally, FPR1−/− differentiated PLBs (dPLBs) were specifically insensitive to fMLF stimulation, but not to LTB4 or C5a, as assessed by the activation of AKT and myosin light chain 2 (MYL2 or MLC) by measuring levels of pAKT and pMLC (Fig. S1F) and by measuring chemotaxis towards fNLFNYK (Fig. S1G). Importantly, we found that fNLFNYK–FITC was readily internalized in Scr dPLBs (Fig. S1H, white arrowheads), but not in FPR1−/− dPLBs, establishing that internalization of the ligand is dependent on FPR1. Furthermore, we found that CRISPR-resistant FPR1–mCherry, when expressed in FPR1−/− PLBs, strongly colocalized with the internalized fNLFNYK-FITC [Fig. S1I; Pearson's correlation coefficient (PCC) of 0.78; white arrowheads], showing that the ligand internalizes with the receptor and that it can be used as a proxy to monitor FPR1 dynamics in neutrophils. Finally, we confirmed that fMLF treatment, but not treatment with C5a, induced substantial internalization of FPR1–EGFP in FPR1−/− PLBs (Fig. S2A), establishing ligand specificity for driving FPR1 internalization.
C-terminal Ser/Thr phosphorylation is presumed to drive FPR1 internalization in a GRK2-dependent manner in neutrophils (Liu et al., 2012). We therefore addressed the direct contribution of the clathrin–dynamin–GRK2 pathway to FPR1 endocytosis in neutrophils. Towards this, we first tested the impact of known clathrin-mediated endocytosis inhibitors, such as monodansyl cadaverine (MDC), phenyl arsine oxide (PAO) and PitStop2, on the internalization of transferrin in PMNs. Indeed, the use of inhibitors to study endocytic modes needs careful consideration, as clathrin inhibitors often block actin polymerization when used at inappropriate concentrations (Dutta and Donaldson, 2012). To select for the right dose of endocytic inhibitors in our studies, we therefore monitored their impact on chemoattractant-induced F-actin polarity and also used a CRISPR-based approach to validate our findings with the inhibitors. As expected, in response to fNLFNYK stimulation, MDC, PAO and PitStop2 largely inhibited internalization of conjugated transferrin, without impacting F-actin distribution (Fig. S2B,C). Importantly, pre-treatment of PMNs with known inhibitors to clathrin (MDC and PAO) and dynamin (OctMAB, MitMAB and Dynasore) did not impact the extent of FPR1 internalization when the inhibitors were used at a concentration where F-actin distribution was not grossly perturbed upon fNLFNYK–FITC stimulation (Fig. 1D,E). Similarly, cells lacking clathrin heavy chain (CHC) did not exhibit FPR1 internalization defects (see below, Fig. 2C,D). Next, we generated GRK2−/− dPLBs (Table S1; Fig. S3A) and, upon treatment with fNLFNYK–FITC, we did not observe any significant difference in the internalization of FPR1 between Scr and GRK2−/− dPLBs (Fig. 1F,G). Furthermore, we assessed the extent of internalization of the FPR1-ΔST mutant, where all Ser and Thr residues in the C-terminal tail are converted into Ala residues (Hsu et al., 1999). We expressed either wild-type (WT) FPR1–mCherry or FPR1-ΔST–mCherry in FPR1−/− dPLBs and observed that both the WT and ΔST mutant cells internalized fNLFNYK–FITC (Fig. S2D,E; blue arrowheads), consistent with our observations in GRK2−/− dPLBs (Fig. 1G). However, the amount of internalized fNLFNYK–FITC in the FPR1-ΔST-expressing dPLBs was significantly lower than that in WT FPR1 cells (Fig. S2E), which correlated with the increased ability of the mutant to chemotax toward fNLFNYK (Fig. S2F). Interestingly, Lui and colleagues have reported that overexpressing the FPR1-ΔST mutant leads to enhanced FPR1-ΔST membrane retention and chemotaxis towards fMLF compared to WT FPR1 overexpression (Liu et al., 2012). We reason that the discrepancy arises from the overexpression of the mutant over and above endogenous FPR1 levels, which leads to potentially skewed signaling, and to the use of saturating or higher amounts of ligand. Overall, our findings establish that the clathrin-mediated pathway and C-terminal tail phosphorylation do not contribute to FPR1 endocytosis in neutrophils.
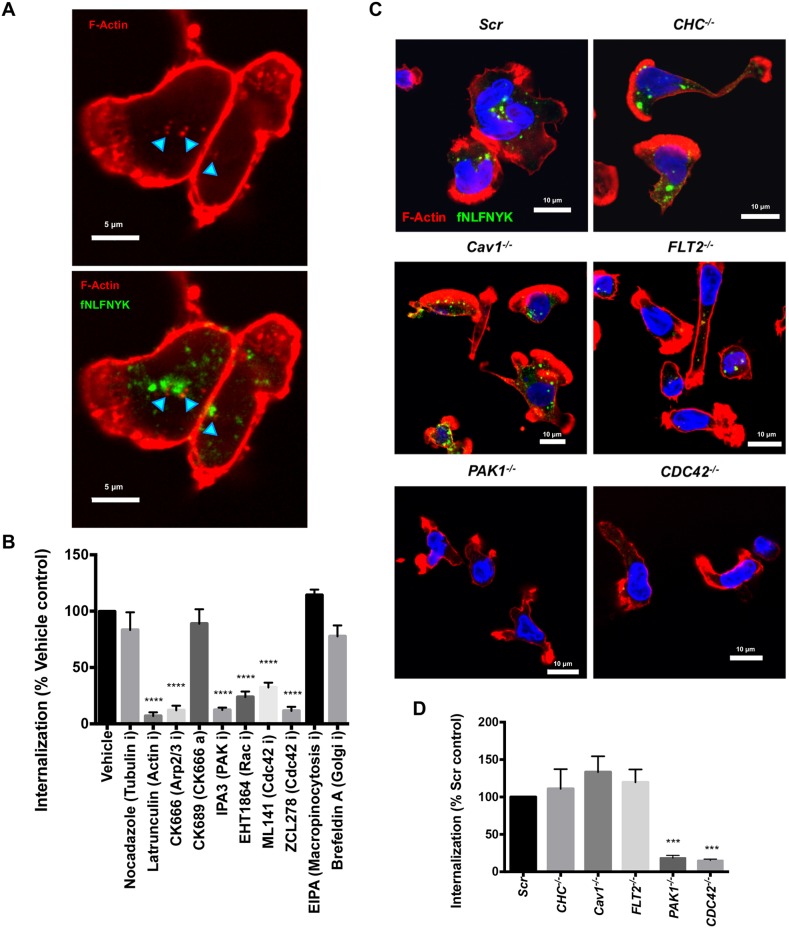
Fig. 2.
FPR1 undergoes CDC42-PAK1-actin-dependent endocytosis. (A) A representative confocal image of PMNs treated with fNLFNYK–FITC (2 nM; green) for 10 min, fixed and stained with phalloidin (red). Turquoise arrowheads highlight the fNLFNYK vesicles enriched with F-actin. Scale bars: 5 µm. Images are representative of at least three independent experiments. (B) Graph depicting the extent of internalization of fNLFNYK–FITC in PMNs pretreated with vehicle [0.2% (v/v) DMSO] or inhibitors or activators (denoted with an ‘i’ and ‘a’ after the protein targeted, respectively) (25 µM nocadazole; 100 nM Lat A; 100 µM CK666; 100 µM CK689; 5 µM IPA3; 25 µM EHT1864; 100 µM ML141; 100 µM ZCL278; 50 µM EIPA; 20 µM Brefeldin A), stimulated with fNLFNYK–FITC (2 nM) for 10 min and fixed. The extent of internalization of fNLFNYK was quantified and represented as the percentage of vehicle control from n=4 independent experiments. Findings are represented as mean±s.e.m. ****P≤0.0001 (one-way ANOVA with Dunnett's multiple comparisons test). (C) Representative confocal images of Scr, CHC−/−, Cav1−/−, FLT2−/−, PAK1−/− and CDC42−/− dPLBs treated with fNLFNYK–FITC (10 nM; green) for 15 min, fixed and stained with phalloidin (red) and DAPI (blue). Scale bars: 10 µm. Images are representative of three independent experiments. (D) Graph depicting the extent of internalization of fNLFNYK–FITC in Scr, CHC−/−, Cav1−/−, FLT2−/−, PAK1−/− and CDC42−/− dPLBs treated with fNLFNYK–FITC (10 nM) for 15 min and fixed. The extent of internalization of fNLFNYK was quantified and represented as a percentage of Scr control from n=4 independent experiments. Findings are represented as mean±s.e.m. ***P≤0.001 (one-way ANOVA with Dunnett's multiple comparisons test).
Both FPR1 and C5aR1 internalization is mediated by the CDC42–actin axis, but they have distinct endocytic fates
We next set out to identify the clathrin and dynamin-independent mode of FPR1 endocytosis in neutrophils (Mayor et al., 2014). We found that the internalized fNLFNYK–FITC-containing endosomes often were enriched on the outside with F-actin (Fig. 2A; cyan arrowheads) and treatment with an F-actin inhibitor (LatA), an Arp2/3 inhibitor (CK666; CK689 was used as an inactive control), a RAC inhibitor (EHT1864), CDC42 inhibitors (ML141 and ZCL278) and a PAK1 inhibitor (IPA3) profoundly impacted F-actin assembly and prevented FPR1 internalization in PMNs (Fig. S3B; Fig. 2B). However, inhibitors of macropinocytosis (EIPA), tubulin polymerization (nocodazole) and Golgi organization (Brefeldin A) hardly influenced FPR1 internalization in PMNs (Fig. S3B; Fig. 2B). We next generated dPLBs lacking endocytic regulators, such as CHC, caveolin 1 (Cav1), flotillin 2 (FLT2, also known as FLOT2), PAK1 or CDC42 (Table S1; Fig. S3A). Consistent with our results with the inhibitors on PMNs, we observed that only cells lacking PAK1 or CDC42 impacted on F-actin assembly and showed profoundly reduced FPR1 internalization in dPLBs (Fig. 2C,D). We conclude that FPR1 is internalized in a CDC42/PAK1-actin-dependent manner upon ligand addition.
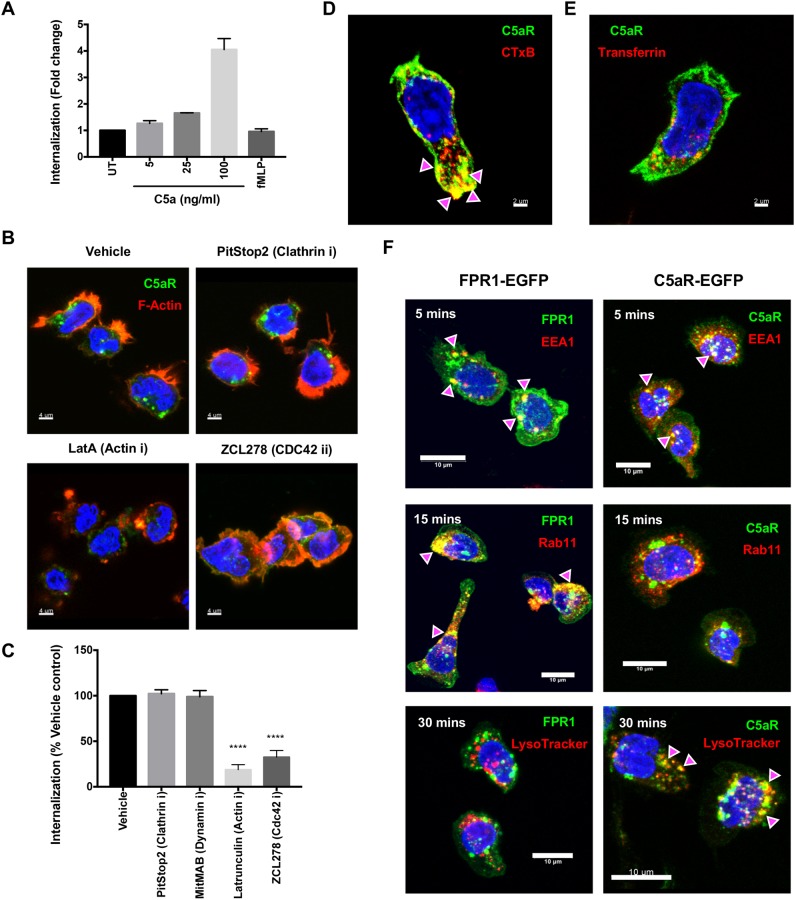
We next studied the internalization mode of another primary chemoattractant GPCR, C5aR1. We expressed C5aR1–EGFP in PLBs and found that 100 ng/ml C5a is required to induce robust endocytosis (Fig. 3A) – a concentration ∼4-fold that of the reported Kd for C5aR1 (∼25 ng/ml) (Chenoweth et al., 1982). Not surprisingly, endocytosis of C5aR1 was specific to C5a, as fMLF treatment did not cause C5aR1 internalization (Fig. 3A). Similar to what is seen with FPR1, endocytosis of C5aR1–EGFP induced by C5a was significantly blocked by inhibitors to actin (LatA) and CDC42 (ZCL278), but not to dynamin (MitMAB) and clathrin (PitStop2) (Fig. 3B,C). Additionally, C5aR1–EGFP colocalized on the plasma membrane with the clathrin-independent cargo CTxB (Fig. 3D; PCC of 0.7, magenta arrowheads), which is also used as a lipid microdomain marker (Mayor et al., 2014), but not with the clathrin-dependent cargo transferrin (Fig. 3E; PCC of 0.46). Thus, the primary chemoattractant GPCRs, FPR1 and C5aR1, internalize as clathrin-independent cargos using the CDC42-actin pathway in neutrophils.
Fig. 3.
C5aR1 internalizes in a clathrin-independent manner and is trafficked to lysosomes. (A) Graph depicting the extent of internalization of C5aR1–EGFP in dPLBs expressing C5aR1–EGFP treated with the indicated doses of C5a or fMLF (10 nM) for 10 min and fixed. Quantification of the extent of C5aR1 internalization is shown relative to untreated control (UT) with n=2 independent experiments and represented as mean±s.d. (B) Representative confocal images of vehicle [0.2% (v/v) DMSO] or inhibitor (denoted with an ‘i’ after the protein targeted) (5 µM PitStop2; 100 nM Lat A; 100 µM ZCL278)-treated C5aR1–EGFP dPLBs (30 min) stimulated with C5a (100 ng/ml) for 15 min, and fixed and co-stained with phalloidin (red) and DAPI (blue). Scale bar: 4 µm. Images are representative of three independent experiments. (C) Graph depicting the extent of internalization of C5aR1 in vehicle or inhibitor-treated C5aR1-EGFP dPLBs (as in panel B and with 5 µM MitMAB), represented as the percentage with respect to vehicle control with n=3 independent experiments. Findings are represented as mean±s.e.m. ****P≤0.0001 (one-way ANOVA with Dunnett's multiple comparisons test). (D,E) Representative multiple intensity projections of Z-stacks for C5aR1–EGFP (green) dPLBs pre-treated with Alexa Fluor 555-conjugated CTxB (1 µg/ml; red; D) or Alexa Fluor 568-conjugated transferrin (5 µg/ml; red; E) for 5 min, and stimulated with C5a (100 ng/ml) for 5 min, fixed and stained with DAPI (blue). Magenta arrowheads represent colocalization on the plasma membrane. Scale bar: 2 µm. Images are representative of two independent experiments. (F) Representative multiple intensity projections of Z-stacks of dPLBs expressing FPR1–EGFP and C5aR1–EGFP treated with fNLFNYK (10 nM) or C5a (100 ng/ml) for the indicated time points, and fixed and co-stained for EEA1 (red; 5 min post stimulation), Rab11 (red; 15 min post stimulation) and LysoTracker (red; 30 min post stimulation). Magenta arrowheads represents colocalization. Scale bar: 10 µm. Images are representative of two independent experiments.
We next addressed the endocytic fates of internalized FPR1 and C5aR1. At 5 min post treatment with their respective ligands, we found that both FPR1 and C5aR1 trafficked to early endosomal compartments marked by EEA1 (Maldonado-Báez et al., 2013) (Fig. 3F; PCC of 0.55 and 0.79, respectively; magenta arrowheads). By 15 min after treatment, internalized FPR1 strongly colocalized with recycling endosomes marked by Rab11a (Maldonado-Báez et al., 2013) (Fig. 3F; PCC of 0.73; magenta arrowheads) and failed to localize to lysosomes (Fig. 3F; PCC of 0.14). In contrast, internalized C5aR1 did not localize to Rab11 compartments (Fig. 3F; PCC of 0.3), but instead strongly localized to lysosomes after 30 min of C5a stimulation (Fig. 3F; PCC of 0.76, magenta arrowheads). The diverging trafficking fates of FPR1 and C5a is consistent with previously reported fractionation-based experiments (Suvorova et al., 2005).
Migrating neutrophils internalize GPCRs from their trailing edge
We next investigated the dynamics of FPR1 and C5aR1 in fast-migrating neutrophils by using time-lapse imaging of dPLBs expressing FPR1–EGFP or C5aR1–EGFP chemotaxing towards a gradient of fNLFNYK or C5a. Strikingly, we found that FPR1–EGFP internalized as clusters from the back of chemotaxing dPLBs (Movie 1). Upon tracking individual vesicles, we observed that the internalized FPR1–EGFP vesicles trafficked directionally up the length of the cell towards the protrusive front (Fig. S4A; track straightness of 0.62). Additionally, similar to what was seen for FPR1–EGFP, we observed clustering and directed trafficking of internalizing C5aR1–EGFP-containing vesicles from the trailing edge of chemotaxing cells (Movie 2, Fig. S4B; track straightness of 0.66). Taken together, these studies establish that primary chemoattractant GPCRs internalize predominantly as clusters from the trailing edge that are trafficked directionally towards the protrusive front in chemotaxing neutrophils (Fig. S4D).
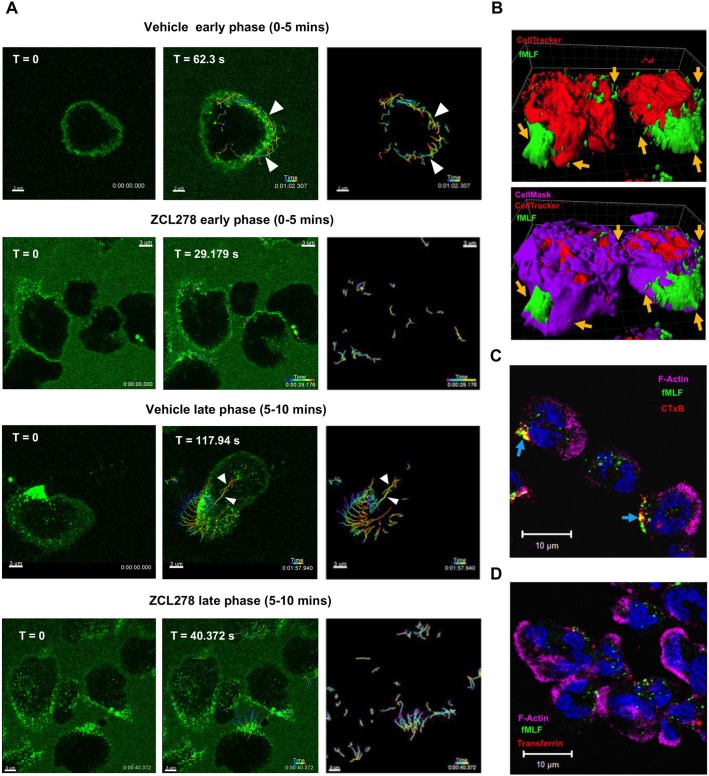
fMLF-bound FPR1 clusters migrate and internalize from lipid microdomains on the plasma membrane at the trailing edge of PMNs
We next studied the spatiotemporal dynamics of FPR1 internalization in PMNs by following the distribution of fMLF–Alexa-Fluor-488 (50 µM) from the time of stimulation. Upon the addition of the ligand, we readily observed uniform ligand binding along the membrane of PMNs (Movie 3 and Fig. 4A; t=0 s in ‘vehicle early phase’). As time progressed, we observed that FPR1 clusters formed and re-distributed (track straightness of 0.548) to the trailing edge of polarizing cells at the early phase (0–5 min) of stimulation (Fig. 4A; t=62.3 s in vehicle early phase, white arrowheads). Importantly, the ability of PMNs to form and re-distribute FPR1 clusters was dependent on CDC42, as we observed far fewer clusters (29 in vehicle versus 5 in ZCL278-treated cells) that re-distributed to the trailing edge of stimulated ZCL278-treated PMNs (Fig. 4A; vehicle and ZCL278 treatments at early phase; Movies 3 and 4). At later time points (5–10 min post stimulation) the directed trafficking of internalized or internalizing FPR1 clusters from the trailing edge to the front of PMNs was readily observed (track straightness of 0.6; Fig. 4A, vehicle late phase, white arrowheads; Movie 3) and again inhibited in ZCL278-treated cells (Fig. 4A, ZCL278 late phase; Movie 4). The response of PMNs to conjugated fMLF was specific as the (1) plasma membrane binding (labeled with CellMask), (2) clustering response, (3) re-distribution to the trailing edge and (4) internalization from the back to the front of PMNs was absent when α3 integrin antibody–Alexa Fluor 488 was added along with stimulation with fNLFNYK (Fig. S4C). Taken together, these findings show that CDC42 is required for FPR1 clustering, re-orientation and directional trafficking of FPR1 in stimulated PMNs.
Fig. 4.
Dynamic clustering, mobilization and endocytosis of ligand-bound FPR1 from lipid microdomains in PMNs. (A) Representative multiple intensity projections and the tracked vesicles across time (right-hand images) of the bottom 2 µm of PMNs pre-treated for 20 min with DMSO (0.2% v/v) or ZCL278 (50 µM) followed by fMLF–Alexa-Fluor-488 (50 nM) stimulation. Images are presented from Movies 3 and 4; t=0 represents the initial time point when vesicles began to be tracked. White arrowheads in the ‘vehicle early phase’ represent the tracks of vesicles that are re-distributed to the back of the polarizing cell. White arrowheads in the ‘vehicle late phase’ represent the tracks of vesicles that trafficked directionally from the back of polarized cell. Scale bars: 3 µm except for the vehicle early phase images where the bar is 2 µm. (B) Representative surface rendering of PMNs pre-stained with CellMask Deep Red (purple) to label the plasma membrane and CellTracker CMPTX Red (red) to label the cytoplasm for 15–20 min before stimulating with fMLF–Alexa-Fluor-488 (green; 50 nM). A Z-stack (∼6 µm from the bottom) was obtained after 5 min and the surface rendered using Imaris. Orange arrows point to clustered FPR1 embedded in the plasma membrane across the length of polarized PMNs. Images are representative of two independent experiments. (C,D) Representative multiple intensity projections of PMNs pre-treated for 5 min with Alexa Fluor 555-conjugated CTxB (1 µg/ml; red in C) or Alexa Fluor 568-conjugated transferrin (5 µg/ml; red in D) followed by stimulation with fMLF–Alexa-Fluor-488 (5 nM; green) for 5 min, and fixed and co-stained with phalloidin (purple) and DAPI (blue). Blue arrows in C represent regions of colocalization of FPR1 and CTxB. Scale bars: 10 µm. Images are representative of three independent experiments.
We next asked whether the FPR1 clusters that re-distribute along the trailing edge of PMNs are membrane-bound or trafficked as internalized vesicles in the cytoplasm. PMNs were co-stained with CellTracker (cytoplasm) or CellMask (plasma membrane) before stimulation with labeled fMLF. Live-imaging of the bottom 5–6 µm of PMNs revealed that the fMLF-bound FPR1 clusters are primarily associated with the plasma membrane and are predominantly at the trailing edge of PMNs (Movie 5 and Fig. 4B, orange arrows). Internalized FPR1 close to the protruding front was also observed, most likely reflecting the trafficked FPR1-containing endosomes in PMNs (Movie 5 and Fig. 4B). Moreover, internalizing FPR1 clusters at an early time point (∼5 min post stimulation) were associated with F-actin-rich regions at the trailing edge of PMNs (Fig. 1C), and colocalized on the plasma membrane with the internalizing lipid-microdomain-dependent and clathrin-independent cargo CTxB (Fig. 4C; PCC of 0.54; blue arrows) on the plasma membrane, but not with the clathrin-dependent cargo transferrin (Fig. 4D, PCC of 0.3). Together, these findings establish that FPR1 clusters and re-distributes to lipid-microdomain-rich regions at the back of polarized neutrophils and internalizes as a CDC42-dependent cargo towards the cell front (Fig. S4D).
BLT1 is resistant to internalization in response to physiological stimulation
We next studied the internalization and dynamics of BLT1 in neutrophils as studies in heterologous cell types point to a clathrin-dependent internalization of BLT1 (Chen et al., 2004; Jala et al., 2005; Subramanian et al., 2017). We first generated BLT1−/− PLBs by expressing sgRNAs that targeted a specific region in the first 250 bps of the coding region of the endogenous BLT1 (Table S1), which resulted in a specific frame shift mutation in the targeted loci in BLT1 (Fig. S5A). We also generated another PLB line with sgRNA (#2) targeting a different portion in the first 250 bps of BLT1 and obtained a frameshift insertion in BLT1 (Table S1; Fig. S5B). Owing to the lack of availability of reliable and specific antibodies to detect endogenous BLT1 from cell lysates, we resorted to functional validation of the BLT1 frameshift mutants (BLT1−/− PLBs). BLT1−/− dPLBs (both sgRNAs) failed to chemotax in response to a LTB4 gradient (Fig. S5C) and displayed reduced pMLC in response to primary chemoattractants such as fMLF and C5a (Fig. S5D) – findings that are consistent with the role of BLT1 in mediating signal relay during neutrophil chemotaxis (Afonso et al., 2012). Furthermore, expression of a CRISPR-resistant BLT1–EGFP in BLT1−/− PLB cells (sgRNA1 clone) rescued the chemotaxis defect in response to an LTB4 gradient (Fig. S5C).
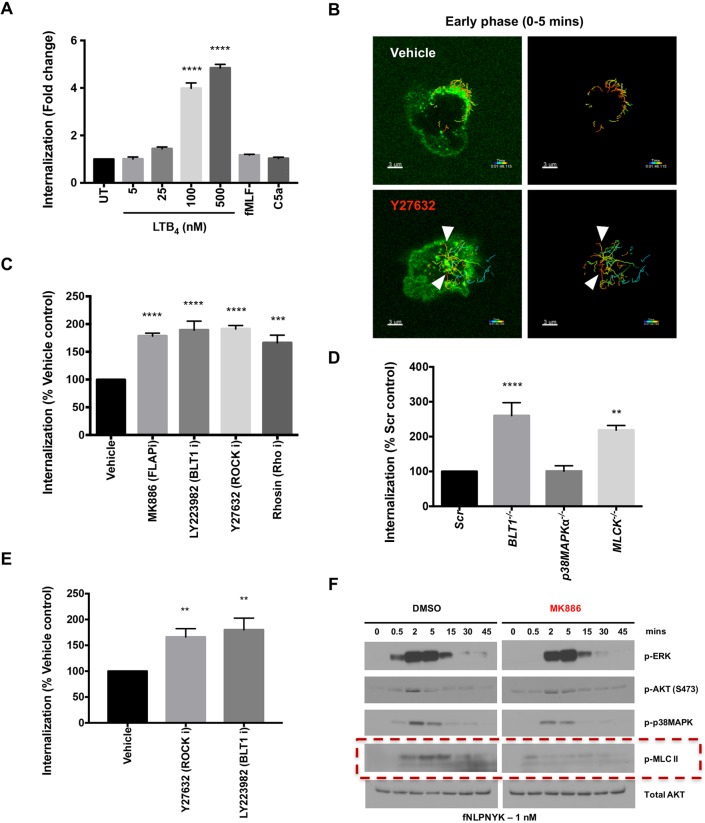
Using the CRISPR-resistant BLT1–EGFP-expressing BLT1−/− PLB cells, we next performed internalization assays. We found a dose- and time-dependent increase in the internalization of BLT1–EGFP in response to LTB4, with 100 nM LTB4 required to induce substantial internalization by 10 min of stimulation (Fig. 5A; Fig. S6A). The reported Kd of BLT1 for LTB4 is ∼1 nM (Gaudreau et al., 2004), suggesting that BLT1 is largely resistant to endocytosis under physiological conditions. Consistent with this, stimulation with the primary chemoattractants fNLFNYK or C5a, which induce LTB4 production (Afonso et al., 2012; Pazos et al., 2015), also failed to induce BLT1–EGFP endocytosis (Fig. 5A; Fig. S6B). However, unlike FPR1 and C5aR1, we found that BLT1 internalization was blocked in the presence of clathrin-dynamin (MDC, PAO, PitStop2, OctMAB and MitMAB) and microtubule (nocodazole) inhibitors, but not actin inhibitor (LatA), in response to non-physiological concentration of LTB4 (Fig. S6C,D). The role of di-leucine motifs in the C-terminal tail of BLT1 in its internalization has been controversial, as expression of a BLT1 mutant with Leu304 and Leu305 mutated to Ala (LL304-5AA or LL/AA mutant) gives rise to higher internalization in CHO cells (Aratake et al., 2012), while the expression of the same mutant in HEK293 cells resisted internalization in response to LTB4 treatment (Gaudreau et al., 2004). To test the role of the di-leucine motifs in regulating BLT1 internalization in neutrophils, we next expressed the LL/AA mutant of BLT1 in BLT1−/− dPLBs. Consistent with the observations in CHO cells, we found that the mutant internalized five times greater than WT BLT1 in response to LTB4 stimulation (Fig. S6E,F). Importantly, the greater internalization observed in cells expressing BLT1 LL/AA-EGFP gave rise to lowered signaling and chemotactic response to LTB4 when compared to cells expressing WT BLT1–EGFP (Figs S6G and S5C). Therefore, a greater receptor retention on the plasma membrane drives persistent signaling and chemotactic signaling in response to LTB4.
Fig. 5.
BLT1 signaling and myosin activation restrict primary GPCR internalization. (A) Graph depicting the extent of internalization of BLT1–EGFP in BLT1−/− dPLBs expressing BLT1–EGFP that were untreated (UT) or treated with the indicated concentration of LTB4, fMLF (10 nM) or C5a (250 ng/ml) for 10 min. The extent of internalization was analyzed as discussed in the Materials and Methods section. Results are presented as the fold change over UT control from n=3 independent experiments and represented as mean±s.e.m. ****P≤0.0001 (one-way ANOVA with Dunnett's multiple comparisons test). (B) Representative multiple intensity projections and the tracked vesicles across time (right-hand images) from live-cell imaging of the bottom 2 µm of PMNs pre-treated for 20 min with either DMSO (0.2% v/v) or Y27632 (25 µM) followed by fMLF–Alexa-Fluor-488 (50 nM) stimulation. White arrowheads represent the tracks of vesicles that trafficked directionally to the center of the cell in the first 5 min of Y27632 treatment. Scale bars: 3 µm. (C) Graph depicting the extent of internalization of fNLFNYK–FITC in PMNs pre-treated with vehicle [0.2% (v/v) DMSO] or inhibitors (denoted with an ‘i’ after the protein targeted) (1 µM MK886, 10 µM LY223982, 25 µM Y27632, 50 µM Rhosin) for 30 min before stimulation with fNLFNYK–FITC (2 nM) for 10 min, and fixed and co-stained with phalloidin and DAPI. The extent of internalization is compared to vehicle control with n=3 independent experiments and represented as mean±s.e.m. ***P≤0.001, ****P≤0.0001 (one-way ANOVA with Dunnett's multiple comparisons test). (D) Graph depicting the extent of internalization of fNLFNYK–FITC in Scr, BLT1−/−, p38MAPK−/− or MLCK−/− dPLBs stimulated with fNLFNYK–FITC (10 nM) for 15 min, and fixed and co-stained with phalloidin and DAPI. The extent of internalization is compared to Scr control with n=4 independent experiments and represented as mean±s.e.m. **P≤0.01, ****P≤0.0001 (one-way ANOVA with Dunnett's multiple comparisons test). (E) Graph depicting the extent of internalization of C5a–EGFP in dPLBs expressing C5aR1–EGFP pre-treated with vehicle [0.2% (v/v) DMSO] or inhibitors (25 µM Y27632, 10 µM LY223982) for 30 min before stimulation with C5a (100 ng/ml), and fixed and co-stained with phalloidin and DAPI. The extent of internalization is compared with vehicle control with n=3 independent experiments and represented as mean±s.e.m. **P≤0.01 (one-way ANOVA with Dunnett's multiple comparisons test). (F) A representative western blot analysis of protein lysates from PMNs that were pre-treated with vehicle [0.2% (v/v) DMSO] or MK886 (1 µM) for 30 min before stimulation with fNLFNYK (1 nM) for indicated time points. Red box highlights the effect of inhibitor treatment on p-MLCII. Images represent results from one of two independent experiments.
Signal-relay and back retraction restrict primary chemoattractant GPCR internalization and promote chemotactic signaling in neutrophils
Next, we studied the role of the RHO-ROCK pathway and retraction of the back of the cell in regulating chemoattractant GPCR internalization. Treatment with the ROCK inhibitor Y27632 did not impact initial FPR1 clustering, although it did impair back retraction (Movie 6). Upon tracking internalizing vesicles over time, we observed that Y27632 treatment promotes the early internalization of FPR1 (by 5 min of stimulation) when compared to vehicle control (Fig. 5B; white arrowheads). Furthermore, inhibition of RHO or ROCK with Rhosin or Y27632, respectively, resulted in a similar enhanced FPR1 internalization 10 min post-stimulation (Fig. 5C). Similar findings were obtained using dPLB cells lacking myosin light chain kinase (MLCK) (Table S1, Fig. S3A; Fig. 5D). Consistent with the results with FPR1, we observed enhanced internalization of C5aR1–EGFP upon Y27632 treatment in dPLBs (Fig. 5E), suggesting that the back retraction pathway in general promotes membrane retention of primary chemoattractant GPCRs. In neutrophils, the LTB4-BLT1 axis potentiates fMLF-mediated myosin activation (Afonso et al., 2012). Indeed, as we previously found (Afonso et al., 2012), blocking LTB4 synthesis (using MK886) leads to a strong inhibition of myosin activation upon fMLF stimulation (Fig. 5F; red dashed box). Importantly, we now find that blocking LTB4 synthesis (MK886) or LTB4 binding to BLT1 (LY223982 or BLT1−/− dPLBs) results in greater internalization of both FPR1 (Fig. 5C,D) and C5aR1 (Fig. 5E), most likely by regulating myosin activation.
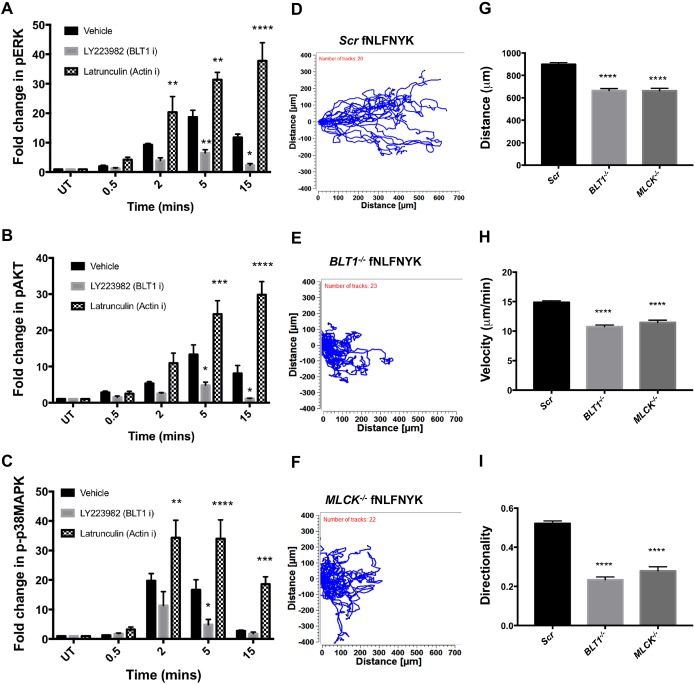
We next investigated how altering the extent of internalization of a primary chemoattractant GPCR impacts downstream signaling. To achieve this, we first assessed the impact of different inhibitors that regulate FPR1 internalization on downstream kinase activation. Treatment of PMNs with LatA, which blocks FPR1 internalization (Fig. 2B), led to a profound increase and sustained kinase activation upon fMLF stimulation (Fig. S7A; Fig. 6A–C). On the other hand, inhibition of BLT1 (LY223982), which enhances FPR1 internalization, resulted in a lower and less sustained activation of kinases in response to fMLF stimulation (Fig. S7A; Fig. 6A–C). Consistent with these negative effects on internalization and signaling, dPLBs lacking BLT1 or MLCK exhibited impaired chemotaxis towards fNLFNYK (Fig. 6D–H) and C5a (Fig. S7B–F). Importantly, the lack of BLT1 or MLCK had the most severe impact on the directionality of chemotaxing cells towards fNLFNYK (Fig. 6I) and C5a (Fig. S7G). Taken together, these findings suggest that signal relay and back retraction contribute to maintaining primary chemoattractant GPCRs on the cell surface for sustained chemotaxis (Fig. 7).
Fig. 6.
The extent of FPR1 internalization impacts downstream signaling and chemotaxis behavior. (A–C) Graph depicting the quantification of pERK (A), pAKT (B) and p-p38MAPK (C) from western blot analyses of the indicated samples (see Fig. S7A) from n=4 independent experiments, represented as mean± s.e.m. ****P≤0.0001, ***P ≤0.001, **P ≤0.01, *P≤0.05, (two-way ANOVA with Dunnett's multiple comparisons test). (D–F) Cell tracks from a single experiment where Scr (D), BLT1−/− (E) or MLCK−/− (F) dPLBs were allowed to migrate in a gradient established from 100 nM of fNLFNYK in an under agarose assay. (G–I) The distance (G), velocity (H) and directionality (I) of Scr, BLT1−/− or MLCK−/− cells migrating in a gradient of fNLFNYK (see D,E). A minimum of 20 cells were manually tracked for each cell line in a single experiment with n=3 independent experiments and results are represented as mean±s.e.m. ****P≤0.0001 (one-way ANOVA involving Dunnett's multiple comparisons test).
Fig. 7.
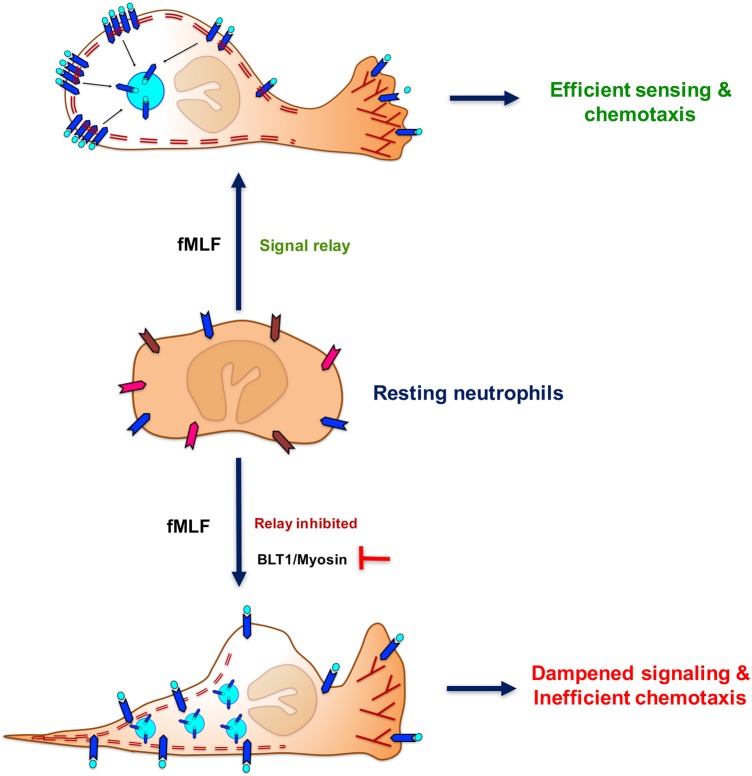
Schematic representation of PMNs internalizing FPR1 from the trailing edge of a neutrophil and the contribution of the BLT1-myosin axis in restricting the extent of FPR1 internalization to promote the directional migration toward fMLF. Elongated chevrons with different colors in the resting neutrophils represent ligand-free GPCRs. The blue chevrons with aqua circles represent fMLF-bound FPR1 in stimulated neutrophils. Double dashed maroon lines represent F-actin networks at the back of the cell. Maroon-colored ‘Y’ shapes represents dynamic and branched F-actin at the protrusive front of a stimulated neutrophil.
DISCUSSION
We set out to identify the mechanisms underlying the internalization and recycling of chemotactic GPCRs in neutrophils. Although internalization of FPR1 and C5aR1 has been explored in heterologous cell culture models (Xue et al., 2004; Vines et al., 2003; Suvorova et al., 2005; Hsu et al., 1999), our study is the most direct and extensive to establish the clathrin-independent and polarized internalization of FPR1 and C5aR1 in neutrophils. Indeed, while the spatial regulation of endocytosis involving clathrin-dependent and -independent pathways has been characterized in specialized and polarized cells, such as epithelial cells (Sandvig et al., 2008; Wirtz-Peitz and Zallen, 2009), hepatic cells (Tuma et al., 2001) and neurons (Itofusa and Kamiguchi, 2011), it had not been reported in fast-migrating neutrophils. In addition, clathrin-independent cargos have been shown to internalize from the leading edge of migrating fibroblasts (Howes et al., 2010). However, unbranched F-actin and lipid-microdomain regions are best-observed along the trailing edge of a polarized neutrophil (Hind et al., 2016), and blocking neutrophil polarity using inhibitors to actin or its regulators dramatically inhibited the internalization of the clathrin-independent cargos FPR1 and C5aR1. Furthermore, we established that internalized FPR1 vesicles are trafficked from the back to the front of chemotaxing cells. Lipid-anchored membrane proteins are known to be internalized in a CDC42-dependent manner (Mayor et al., 2014). CDC42 is also known to promote trailing edge stability in neutrophils aside from its proposed role as a compass in steering neutrophil migration (Yang et al., 2016; Hind et al., 2016). Moreover, FPR1 clusters have been shown to localize to ganglioside monoside 1-enriched lipid-microdomains in U937 cells (Xue et al., 2004; Mayor et al., 2014). Therefore, blocking CDC42 or F-actin not only prevents neutrophils from polarizing, but most likely blocks lipid-microdomain formation, which is essential for clathrin-independent endocytosis to occur from the trailing edge in neutrophils. Interestingly, unlike clathrin-independent cargos, we found that BLT1 internalization via clathrin is not dependent on cell polarity.
The spatio-temporal dynamics of chemoattractant GPCRs on the plasma membrane of neutrophils has largely remained unexplored. Through live imaging of fluorescence-labeled ligands, we observed two phases of FPR1 dynamics on neutrophils. In the early stages (0–5 min), FPR1 clusters form on the plasma membrane and they re-distribute, as clusters, towards the trailing edge of polarizing cells – these events happen in relatively stationary cells. At later times (5–10 min), neutrophils are completely polarized and FPR1 clusters internalizing from the back of cells are trafficked towards the protruding front – these events occur when stimulated neutrophils begin to migrate. Under these conditions, we did observe that the fluorescence labeling on the plasma membrane remains low and constant throughout the acquisition time and that receptor clusters on the plasma membrane, and subsequently in the internalized vesicles, have higher fluorescence intensity than the rest of the plasma membrane. Live imaging was carried out in the presence of fluorescent fMLF throughout the acquisition period, and therefore conjugated ligand was always accessible to unoccupied FPR1, which may contribute to the uniform membrane labeling seen throughout the movie. The dynamic change in PMN morphology is associated with dynamic changes to the plasma membrane. Given that we could only image 2–3 µm from the bottom of the cell, we were unable to directly visualize the movement of fMLF-bound FPR1 in and out of the imaging area. We also failed to observe a strong uniform membrane-bound fMLF signal on the plasma membrane of fixed cells. However, receptor clusters or internalized FPR1-containing vesicles were strongly detected in both live and fixed cells. These findings suggest that the low, uniform conjugated fMLF labeling on the plasma membrane represents dynamic fMLF–FPR1 interactions and that receptor clusters represent stronger fMLF–FPR1 interactions that eventually get internalized from the trailing edge of polarized PMNs. We envisage that there is a similar response by the other clathrin-independent CDC42-dependent cargo, C5aR1, in neutrophils.
The chemotactic responses of the mutants of FPR1 and BLT1, that impact GPCR internalization in opposite ways, revealed a key role for the endocytosis of chemoattractant GPCRs in the chemotactic response of neutrophils. Cells expressing the FPR1-ΔST mutant showed a significantly reduced internalized FPR1 pool and a greater chemotactic response compared to cells expressing WT FPR1. Conversely, expression of the BLT1 mutant (LL/AA), which internalized significantly more than WT BLT1, gave rise to reduced signaling and chemotactic responses to LTB4. Therefore, membrane retention of GPCRs supports a robust chemotactic response. Interestingly, we also found that the fate of trafficked GPCRs regulates chemotactic responses. Heit and colleagues reported that, while the primary chemoattractants fMLF and C5a induce equivalent chemotactic responses, the amount of fMLF required to do so is much less compared to the amount of C5a (Heit et al., 2002). Moreover, at equivalent concentrations, fMLF promoted neutrophils to migrate a longer distance compared to C5a (Foxman et al., 1997). We now find that while FPR1 is trafficked to recycling endosomes, C5aR1 is trafficked for degradation to lysosomes. It therefore appears that endosomal recycling of FPR1 and lysosomal trafficking of C5aR1 correlate with the extent to which neutrophils chemotax to fMLF and C5a.
The relationship between the spatial distribution of chemoattractant GPCRs on the plasma membrane and the receptor activity driving persistent neutrophil chemotaxis is not well characterized. Increased membrane folds at the protruding front are thought to provide access to a greater number of GPCRs to the available chemoattractant (Servant et al., 1999). However, such a concept does not account for GPCR desensitization via internalization, and fails to explain why steep signaling gradients are established across the length of a chemotaxing neutrophil (Cai and Devreotes, 2011; Janetopoulos and Firtel, 2008). While our findings have not directly addressed chemoattractant GPCR desensitization involving modifications by GRKs or other kinases to mediate stop signals, our study does provide novel insight into the dynamic clustering and the spatial re-distribution of ligand-bound GPCRs along the membrane of the trailing edge of stimulated neutrophils. While the continuous ligand-bound GPCRs at the protruding front most likely promote sustained receptor signaling (Cai and Devreotes, 2011; Janetopoulos and Firtel, 2008), clustered GPCRs internalizing from the trailing edge may eventually desensitize and thereby help generate a steep signaling gradient across the length of a migrating neutrophil. Such a concept could support the idea of an indirect amplification of local excitation signals at the protruding front with chemoattractant accessible GPCRs – ideas that resonate with the proposed ‘Local Excitation Global Inhibition’ (LEGI) model (Cai and Devreotes, 2011; Janetopoulos and Firtel, 2008). The concept of polarized internalization and orientated trafficking of cargos within neutrophils has been proposed for recycling integrins (Pierini et al., 2000; Lawson and Maxfield, 1995). However, we provide the first direct visual evidence of such trafficking for chemoattractant GPCRs in neutrophils. The dynamic actin polymerization at the front provides the necessary physical force for cells to push forward up a gradient (Stephens et al., 2008; Leithner et al., 2016), which causes F-actin to eventually flow rearward, sweeping membrane-bound particles to the trailing edge, where contractile forces are thought to drive endocytosis (Mellman, 2000; Stephens et al., 2008). Once internalized, endocytic vesicles undergo oriented recycling to the plasma membrane to supplement the membrane requirement at the protruding cell front (Stephens et al., 2008). Clearly, endocytosis at the protrusive front is physically unfavorable (Diz-Muñoz et al., 2013; Dai and Sheetz, 1995) and likely biologically counterproductive in neutrophils.
Primary chemoattractants such as fMLF and C5a give rise to low, yet sufficient amounts of LTB4, which then acts as a signal-relay molecule during neutrophil chemotaxis. Our study has therefore focused on the dynamics and crosstalk between primary chemoattractant GPCRs, FPR1 and C5aR1, that rely on the secondary chemoattractant GPCR, BLT1, to relay chemotactic signaling and not other GPCRs, such as the CXCL8 receptors, which do not relay chemotactic signals (Afonso et al., 2012). We show that BLT1 helps sustain signaling responses to primary chemoattractants, largely owing to its ability to restrict the internalization of primary chemoattractant GPCRs, as well as its own internalization. By doing so, BLT1 potentially contributes to a better detection and response to small bursts of LTB4, as is predicted with the exosomal packaging and secretion of LTB4 by migrating leader cells (Majumdar et al., 2016). Although F-actin is required for the internalization of clathrin-independent cargos, the precise contribution of myosin II activity to cargo uptake remains unclear (Mayor et al., 2014). In neutrophils, BLT1-mediated myosin II activation appears to prevent GPCR internalization, most likely by promoting back retraction in response to primary chemoattractants. We envision that, by indirectly preventing primary GPCR internalization, BLT1 promotes downstream signaling to sustain neutrophil chemotaxis. Taken together, our findings establish the polarized dynamics of primary chemoattractant GPCRs and their crosstalk with the LTB4–BLT1 axis in promoting chemotactic sensing, which underlies neutrophil and other such innate immune cellular responses to chemotactic agents.
MATERIALS AND METHODS
Reagents
Dimethyl sulfoxide (D2650), Histopaque 1077, saponin, BSA (A9576), PitStop 2, phenylarsine oxide (PAO; P3075), monodansyl cadaverine (MDC; 30432), methyl-β-cyclodextrin (C4555), Filipin (F4767), CK666, CK689, 5-(N-Ethyl-N-isopropyl)amiloride (EIPA), 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; D8417) and Brefeldin A (B5936) were purchased from Sigma Aldrich (St Louis, MO). The formyl-Nle-Leu-Phe-Nle-Tyr-Lys fluorescein derivative (fNLFNYK–FITC), Rhodamine–phalloidin, Alexa Fluor 647-conjugated phalloidin, Alexa Fluor 568-conjugated transferrin from human serum, Alexa Fluor 555-conjugated Cholera toxin B subunit (CTxB), CellTracker Red CMPTX dye, CellMask Deep Red Plasma membrane stain and LysoTracker Red DND-99, Alexa Fluor 568-conjugated donkey antibodies to IgG (mouse, rabbit and goat) and Latrunculin A (LatA) were obtained from Molecular Probes. OctMAB, MitMAB, Dynasore, IPA3, EHT1864, ML141, ZCL278, Rhosin and MK886 were obtained from Tocris Bioscience. LTB4, LY223982 and Y27632 were from Cayman Chemical. fMLF–Alexa-Fluor-488 was synthesized by Discovery Peptides, Cambridge Research Biochemicals. C5a was obtained from R&D Biosystems. Nocadazole was purchased from Calbiochem.
Primary neutrophil isolation
Heparinized whole blood from anonymous healthy human donors was obtained by venipuncture from the NIH Blood Bank research program. Neutrophils were isolated by dextran sedimentation (3% dextran in 0.9% NaCl solution) followed by overlaying onto Histopaque 1077 and differential centrifugation as reported previously (Afonso et al., 2012). Neutrophils were cleared off erythrocytes by multiple rounds of hypotonic lysis with 0.2% NaCl solution followed by neutralization with 1.6% NaCl solution.
Cell lines and constructs
Human leukemic cell line PLB-985 cells were cultured in RPMI medium 1640 – GlutaMAX (Gibco) supplemented with 10 µM HEPES (Gibco), 100 U/ml of pencillin-streptomycin mixture (Gibco) and 10% heat-inactivated FBS (Gemini Bio Products). All the cell lines used and generated from PLB-985 cells were periodically tested for mycoplasma contamination, which was confirmed to be negative in this study. PLBs were differentiated as described previously (Majumdar et al., 2016) using 1.3% DMSO every 48 h for a period of 6 days. The DNA templates for FPR1 and FPR1-ΔST mutant were a kind gift from Dr Richard Ye (University of Macau, Institute of Chinese Medical Sciences). The BLT1 template was from Origene. FPR1 and BLT1 were tagged with EGFP- or mCherry-expressing sequence at the C-terminus and cloned into the pMSCVneo vector (Clontech Laboratories). C5aR1–EFGP was amplified from Addgene plasmid #62612 (deposited by Heini Miettinen; Suvorova et al., 2005). pMSCV-based constructs were transfected using Lipofectamine 2000 (Invitrogen) into Phoenix 293T packaging cells to produce retroviruses, which were then used to infect and select PLB cells. All the experiments in this study were performed in RPMI without Phenol Red and at 37°C and 5% CO2.
CRISPR-based knockouts and expression of CRISPR-resistant GPCRs
The nucleotide sequence in the human genomic DNA (gDNA) that transcribes the first 250 bps of the first exon of the target protein was used to obtain potential sgRNAs from the CRISPR Design tool (http://crispr.mit.edu/) developed by Dr Feng Zhang's group at MIT, Boston. The sgRNAs with the highest scores from the CRISPR Design tool were used in this study. A scrambled (Scr) sgRNA was also designed using the siRNA Wizard 3.1 tool (InvivoGen) that does not target any exon in the human gDNA using sgRNA with the highest score that targets the FPR1 locus as a template. A list of sgRNA sequences targeting specific proteins is listed in Table S1. sgRNAs encoding sequences were cloned into plentiCRISPRv2 as described by Dr Feng Zhang's group (Addgene plasmid #52961, deposited by Feng Zhang, Sanjana et al., 2014). pLentiCRISPRv2 constructs were transfected into 293T cells by using the Trans-Lentiviral shRNA packaging kit (Thermo Scientific; # TLP5913) to produce lentiviruses that were then used to infect PLB cells. Transfected PLB cells were selected with puromycin (2.5 µg/ml), propagated and sub-cloned at ∼2–3 cells/well. Cells from each well were considered as single clones in this study and, wherever possible, each clone was subjected to western blot analysis to detect loss of target protein expression and/or subjected to functional assays to shortlist potential knockout clones. In case of PLB clones targeting the FPR1 and BLT1 loci, owing to the lack of specific antibodies, functional responses such as activation of AKT, ERK or MLC were used to shortlist potential knockout clones. The gDNA from shortlisted clones was extracted, and then the target loci and the top two potential exonic off-target loci were PCR amplified and sequenced for indels. Successful clones were found to have a frameshift mutation (deletion or insertion) at the target site but not at potential off-target sites. FPR1–EGFP, FPR1ΔST–EGFP, BLT1–EGFP and BLT1LL/AA–EGFP resistant to CRISPR activity were generated by introducing in-frame silent mutations into the PAM site (NGG) using site-directed mutagenesis, and were transfected into FPR1−/− and BLT1−/− PLBs, respectively.
Uniform stimulation internalization assay and vesicle tracking
For the analysis of internalization, polymorphonuclear neutrophils (PMNs) or dPLBs were seeded at 0.1×106–0.2×106 cells in each well of an 8-well chambered slide (Lab-TekII; Thermo Fisher Scientific) coated with 1% BSA in Dulbecco's phosphate-buffered saline (DPBS; Gibco) overnight. Wherever necessary, PMNs were pre-treated with specific inhibitors for at least 30 min before stimulation. For internalization of GPCRs fNLFNYK–FITC or fMLF–Alexa-Fluor-488 was used to track FPR1. dPLBs expressing BLT1–EGFP or C5aR1–EGFP were used to analyze internalization of BLT1 with LTB4 or C5aR1 with C5a, respectively. At a given time point, cells were washed and adherent cells were fixed with 4% paraformaldehyde solution (Electron Microscopy Sciences) for ∼4–5 min at 37°C. Once fixed, cells were permeabilized with 0.05% saponin in 3% BSA-containing DPBS and stained with Rhodamine–phalloidin (0.2 Units) and DAPI (10 ng/ml). Cells were imaged using a 40× objective lens on a Zeiss observer microscope (Zeiss Axiovert S100 microscope) or Revolve FL (FJSD1000) microscope from ECHO Laboratories. Cells in four to six fields across the width of a well were captured randomly for each condition in an experiment. For the total fluorescence intensity measurements, individual cells were identified and measured using the Fiji region of interest (ROI) manager. The background was subtracted using unstained cells as control and the measurements were represented as fold change with respect to the control condition in the experiment. For the spot-detection analysis of internalization, we have used a two-step criteria – size (0.5 µm) and intensity – of spots as detected by the Imaris spot detection method to quantify the extent of internalization. The threshold for the intensity of spots was set manually based on the spots observed in the control condition in each experiment as the exposure settings were identical between conditions in each experiment. FITC or EFGP spots of 500 nm were identified over and above a threshold fluorescence intensity set for the control (vehicle or Scr) sample in each experiment. Spots detected along the plasma membrane (detected using phalloidin staining and membrane EGFP signals) were manually excluded, and spots observed within the cytoplasm were only considered for the analysis of internalization. A total of 100 or more cells were analyzed per condition per experiment, with DAPI staining used to identify the number of cells in each field. The number of spots per cell were averaged for each treatment, and is represented as percentage or as fold change in comparison to an appropriate control. Vesicles (0.5 µm) were tracked using Imaris spot detection over time and represented in figures as cylindrical tracks (>3 µm in length in the time interval analyzed) with a color code-matching time scale. Statistics on the number of tracks and track straightness were also obtained.
Live-cell confocal imaging
For certain experiments, dPLBs expressing EGFP-tagged GPCRs were imaged live using a 60× objective lens on the Carl Zeiss LSM 880 microscope as cells migrated under agarose up a chemoattractant gradient (see below for details of the under agarose assay). Based on the speed of cell movement (10–15 µm/min) and the rate of acquisition, only the bottom 2–3 µm of cells, where most endocytic events could be observed, was reliably and continuously acquired. For certain experiments, PMNs were pre-treated with DMSO or specific inhibitors for 15–20 min before simultaneous stimulation with fMLF–Alexa-Fluor-488, images from the bottom 2–3 µm were continuously acquired. For specific experiments, PMNs were pre-stained with CellMask and CellTracker Red for 20 min, before washing, and stimulation with fMLF–Alexa-Fluor-488 (10 nM or 25 nM) or α3 integrin antibody–Alexa Fluor 488 (Santa Cruz Biotechnology; clone A-3; 1 µg/ml) for indicated times. The bottom 5–6 µm of cells was acquired live and, wherever necessary, the surfaces were rendered using Imaris.
Under agarose assay and chemotaxis analysis
Briefly, 0.5% SeaKem ME agarose (Lonza) in 50% each of PBS and RPMI without Phenol Red was allowed to solidify in 1% BSA-coated six-well plates (In Vitro Scientific, California). Three 2 mm diameter wells were carved out at a 2 mm distance from each other. dPLBs were stained with CellTracker Red CMPTX dye according to the manufacturer's protocol (Life Technologies) and re-suspended in RPMI without Phenol Red. 10 µl (5×104 cells) of stained Scr or BLT1−/− or MLCK−/− cells were added to the wells at the edges and cells were allowed to migrate towards the center well containing 10 µl of either fNLFNYK (100 nM) or 2.5 µg/ml of C5a. Time-lapse imaging was performed using a 10× objective lens (Zeiss Axiovert S100 microscope) at an interval of 45 s between frames for a total period of 60 min in an enclosure set to 37°C and supplied with 5% CO2. Image sequences from under agarose assays were used to manually track 20 or more cells per condition in each experiment using the Manual Tracking plugin for ImageJ. The output files containing the x and y coordinates were then used to obtain the accumulated distance, velocity and directionality of cells using the Chemotaxis plugin (ibidi) available for ImageJ. To identify the number of migrated cells, the Imaris spot detection tool was used at the end of 30 min of imaging for different conditions.
Immunofluorescence and confocal imaging
dPLBs expressing EGFP-tagged GPCR were stimulated and fixed at desired time points with 4% PFA for 5 min at 37°C. After washing, cells were permeabilized using 0.05% saponin in 3% BSA-containing DPBS for 20 min at 37°C before addition of primary antibody (1:100) and incubation at 4°C overnight. Cells were washed and stained with secondary antibody conjugated with Alexa Fluor 568 (1:500) and DAPI (10 ng/ml). For experiments involving LysoTracker (100 nM) or transferrin–Alexa-Fluor-568 (5 µg/ml) or CTxB–Alexa-Fluor-555 (1 µg/ml), dPLBs expressing EGFP-tagged GPCRs were pre-treated with one of the conjugated molecules for 5 min before stimulation with a chemoattractant for a specific time period, followed by fixation and co-staining with DAPI. For certain experiments, PMNs were pre-treated with transferrin–Alexa-Fluor-568 (5 µg/ml) or CTxB–Alexa-Fluor-555 (1 µg/ml) for 5 min before stimulation with fNLFNYK–FITC or fMLF–Alexa-Fluor-488 for specific time periods followed by fixation and co-staining with phalloidin and DAPI. For certain experiments, PMNs were pre-treated with CellTracker Red and CellMask Deep Red for 15 min before adding α3 integrin antibody–Alexa Fluor 488 and stimulating with fNLFNYK (25 nM). In specific experiments, PMNs were treated with β2 integrin antibody–Alexa Fluor 488 (Santa Cruz Biotechnology; clone CTB104) and CellMask Deep Red for 5 min before stimulating with fMLF and were fixed after 10 min of stimulation. To quench membrane-bound Alexa Fluor 488 signals, anti-Alexa Fluor 488 functional antibody (Invitrogen; rabbit oligoclonal; 710369; 1 µg/ml) was added after cell fixation and incubated for at least 30 min in room temperature before further processing. Post-processing, cells were imaged at 60× objective using either a Leica TCS SP8 confocal microscope or a Carl Zeiss LSM 880 with Airyscan microscope. Images were further processed using LAS X software or Imaris to be presented in figures.
Western blot analysis and quantification
PMNs were pretreated with inhibitors for 30 min and, at specific time intervals, cells were lysed by the addition of Laemmli buffer (Bio-Rad Laboratories) containing complete EDTA-free protease inhibitor cocktail (Roche) and PhosSTOP phosphatase inhibitor cocktail (Roche), as suggested by the manufacturer. Lysates were analyzed on 4–12% gradient pre-cast polyacrylamide gels (Bio-Rad Laboratories), transferred to PVDF membranes (Millipore) and blocked with 3% BSA (Fraction V; MP Biomedicals) in Tris-buffered saline solution (Corning) containing 0.05% Tween-20 (Sigma; TBST). fNLFNYK and antibodies to endophilin A2 (EndoA2; sc-365704), flotillin 1 (FLT1; sc-25506), caveolin 1 (Cav1; sc-53564), GRK2 (sc-562) and GAPDH (sc-47724) were from Santa Cruz Biotechnology. Antibodies to p-AKT (Ser473; # 4060), p-ERK (Thr202/Tyr204; # 4370), p-p38MAPK (Thr180/Tyr182; # 4511), p-MLC2 (Ser19; # 3675), AKT (# 9272), caveolin 1 (# 3267), clathrin heavy chain (# 4796), EEA1 (# 3288) and Rab11 (# 5589) were from Cell Signaling Technology. Primary antibodies were diluted according to manufacturer's recommendations (typically 1:1000) in TBST with 1% BSA and incubated with the membrane on a rocking platform overnight. After three successive washes, secondary antibodies conjugated to peroxidase (Jackson Laboratories) were added at 1:5000 dilution for 1 h at room temperature. Blots were developed using a chemiluminescence reagent (GE Healthcare). Band intensity was quantified using the Gel Analyzer tool in ImageJ (NIH) and represented as the fold change with respect to the unstimulated control in each experiment.
Statistical analysis
Data generated using Microsoft Excel were plotted using the Prism 7 tool. For analysis of significance, one-way analysis of variance (ANOVA) with a Dunnett’s multiple comparisons test was carried out for most experiments using Prism 7. For experiments with two conditions, a non-parametric paired Welch's two-tailed t-test was performed. For western blot analysis experiments, significance was analyzed using two-way ANOVA with Dunnett's multiple comparisons test in Prism 7. For analyzing colocalization, Imaris was used to determine the Pearson's correlation coefficient (PCC) on confocal images of cells involving multiple Z-stacks. Importantly, as suggested previously, PCC values were compared between appropriate markers (Dunn et al., 2011), and a PCC of 0.5 or higher was considered substantially colocalized.
Supplementary Material
Acknowledgements
We wish to thank Dr Richard Ye for providing the FPR1 and FPR1-ΔST mutant, and all the members of the Parent laboratory for thoughtful and supportive discussions. Drs Roberto Weigert, Paul Randazzo, Julie Donaldson and Philip Murphy are thanked for their critical and constructive comments as well as for sharing reagents to complete this work. Daniel Letwin and Laquaundra Hampton are acknowledged for their technical assistance. Langston Lim at the CCR Microscopy Core is acknowledged for his technical assistance in image acquisition and analysis. Microscopy Core of LCMB as well as the CCR DNA sequencing and the FACS cores are thanked for all their help in this work.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: B.C.S., K.M., C.A.P.; Methodology: B.C.S., K.M.; Validation: B.C.S., C.A.P.; Formal analysis: B.C.S, K.M.; Investigation: B.C.S.; Writing - original draft: B.C.S.; Writing - review & editing: B.C.S., K.M., C.A.P.; Visualization: B.C.S., C.A.P.; Supervision: C.A.P.; Funding acquisition: C.A.P.
Funding
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.217422.supplemental
References
- Afonso P. V., Janka-Junttila M., Lee Y. J., Mccann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T. and Parent C. A. (2012). LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079-1091. 10.1016/j.devcel.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratake Y., Okuno T., Matsunobu T., Saeki K., Takayanagi R., Furuya S. and Yokomizo T. (2012). Helix 8 of leukotriene B4 receptor 1 inhibits ligand-induced internalization. FASEB J. 26, 4068-4078. 10.1096/fj.12-212050 [DOI] [PubMed] [Google Scholar]
- Artemenko Y., Lampert T. J. and Devreotes P. N. (2014). Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell. Mol. Life Sci. 71, 3711-3747. 10.1007/s00018-014-1638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Christophe T. and Boulay F. (2003). Phosphorylation of key serine residues is required for internalization of the complement 5a (C5a) anaphylatoxin receptor via a beta-arrestin, dynamin, and clathrin-dependent pathway. J. Biol. Chem. 278, 4277-4285. 10.1074/jbc.M210120200 [DOI] [PubMed] [Google Scholar]
- Cai H. and Devreotes P. N. (2011). Moving in the right direction: how eukaryotic cells migrate along chemical gradients. Semin. Cell Dev. Biol. 22, 834-841. 10.1016/j.semcdb.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Gaudreau R., LE Gouill C., Rola-Pleszczynski M. and Stanková J. (2004). Agonist-induced internalization of leukotriene B(4) receptor 1 requires G-protein-coupled receptor kinase 2 but not arrestins. Mol. Pharmacol. 66, 377-386. 10.1124/mol.104.001206 [DOI] [PubMed] [Google Scholar]
- Chen K., Bao Z., Gong W., Tang P., Yoshimura T. and Wang J. M. (2017). Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 85, 64-77. 10.1016/j.jaut.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Goodman M. G. and Weigle W. O. (1982). Demonstration of a specific receptor for human C5a anaphylatoxin on murine macrophages. J. Exp. Med. 156, 68-78. 10.1084/jem.156.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M. and Claing A. (2009). G protein-coupled receptors stimulation and the control of cell migration. Cell. Signal. 21, 1045-1053. 10.1016/j.cellsig.2009.02.008 [DOI] [PubMed] [Google Scholar]
- Dai J. and Sheetz M. P. (1995). Regulation of endocytosis, exocytosis, and shape by membrane tension. Cold Spring Harb. Symp. Quant. Biol. 60, 567-571. 10.1101/SQB.1995.060.01.060 [DOI] [PubMed] [Google Scholar]
- Diz-Muñoz A., Fletcher D. A. and Weiner O. D. (2013). Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23, 47-53. 10.1016/j.tcb.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., Kamocka M. M. and Mcdonald J. H. (2011). A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300, C723-C742. 10.1152/ajpcell.00462.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D. and Donaldson J. G. (2012). Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell Logist 2, 203-208. 10.4161/cl.23967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman E. F., Campbell J. J. and Butcher E. C. (1997). Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139, 1349-1360. 10.1083/jcb.139.5.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau R., Beaulieu M.-E., Chen Z., LE Gouill C., Lavigne P., Staňková J. and Rola-Pleszczynski M. (2004). Structural determinants regulating expression of the high affinity leukotriene B4 receptor: involvement of dileucine motifs and alpha-helix VIII. J. Biol. Chem. 279, 10338-10345. 10.1074/jbc.M309207200 [DOI] [PubMed] [Google Scholar]
- Gilbert T. L., Bennett T. A., Maestas D. C., Cimino D. F. and Prossnitz E. R. (2001). Internalization of the human N-formyl peptide and C5a chemoattractant receptors occurs via clathrin-independent mechanisms. Biochemistry 40, 3467-3475. 10.1021/bi001320y [DOI] [PubMed] [Google Scholar]
- He H.-Q. and Ye R.-D. (2017). The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules 22, 455 10.3390/molecules22030455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit B., Tavener S., Raharjo E. and Kubes P. (2002). An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159, 91-102. 10.1083/jcb.200202114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind L. E., Vincent W. J. B. and Huttenlocher A. (2016). Leading from the back: the role of the uropod in neutrophil polarization and migration. Dev. Cell 38, 161-169. 10.1016/j.devcel.2016.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes M. T., Kirkham M., Riches J., Cortese K., Walser P. J., Simpson F., Hill M. M., Jones A., Lundmark R., Lindsay M. R. et al. (2010). Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 190, 675-691. 10.1083/jcb.201002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. H., Chiang S. C., Ye R. D. and Prossnitz E. R. (1999). Phosphorylation of the N-formyl peptide receptor is required for receptor internalization but not chemotaxis. J. Biol. Chem. 272, 29426-29429. 10.1074/jbc.272.47.29426 [DOI] [PubMed] [Google Scholar]
- Itofusa R. and Kamiguchi H. (2011). Polarizing membrane dynamics and adhesion for growth cone navigation. Mol. Cell. Neurosci. 48, 332-338. 10.1016/j.mcn.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Jala V. R., Shao W.-H. and Haribabu B. (2005). Phosphorylation-independent beta-arrestin translocation and internalization of leukotriene B4 receptors. J. Biol. Chem. 280, 4880-4887. 10.1074/jbc.M409821200 [DOI] [PubMed] [Google Scholar]
- Janetopoulos C. and Firtel R. A. (2008). Directional sensing during chemotaxis. FEBS Lett. 582, 2075-2085. 10.1016/j.febslet.2008.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M. A. and Maxfield F. R. (1995). Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75-79. 10.1038/377075a0 [DOI] [PubMed] [Google Scholar]
- Leithner A., Eichner A., Müller J., Reversat A., Brown M., Schwarz J., Merrin J., de Gorter D. J. J., Schur F., Bayerl J. et al. (2016). Diversified actin protrusions promote environmental exploration but are dispensable for locomotion of leukocytes. Nat. Cell Biol. 18, 1253-1259. 10.1038/ncb3426 [DOI] [PubMed] [Google Scholar]
- Liu X., Ma B., Malik A. B., Tang H., Yang T., Sun B., Wang G., Minshall R. D., Li Y., Zhao Y. et al. (2012). Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 13, 457-464. 10.1038/ni.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar R., Tavakoli Tameh A. and Parent C. A. (2016). Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 14, e1002336 10.1371/journal.pbio.1002336 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maldonado-Báez L., Williamson C. and Donaldson J. G. (2013). Clathrin-independent endocytosis: a cargo-centric view. Exp. Cell Res. 319, 2759-2769. 10.1016/j.yexcr.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S., Parton R. G. and Donaldson J. G. (2014). Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 6, a016758 10.1101/cshperspect.a016758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. (2000). Quo vadis: polarized membrane recycling in motility and phagocytosis. J. Cell Biol. 149, 529-530. 10.1083/jcb.149.3.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M. A., Pirzai W., Yonker L. M., Morisseau C., Gronert K. and Hurley B. P. (2015). Distinct cellular sources of hepoxilin A3 and leukotriene B4 are used to coordinate bacterial-induced neutrophil transepithelial migration. J. Immunol. 194, 1304-1315. 10.4049/jimmunol.1402489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini L. M., Lawson M. A., Eddy R. J., Hendey B. and Maxfield F. R. (2000). Oriented endocytic recycling of alpha5beta1 in motile neutrophils. Blood 95, 2471-2480. [PubMed] [Google Scholar]
- Rieu P., Lesavre P. and Halbwachs-Mecarelli L. (1993). Evidence for integrins other than beta 2 on polymorphonuclear neutrophils: expression of alpha 6 beta 1 heterodimer. J. Leukoc. Biol. 53, 576-582. 10.1002/jlb.53.5.576 [DOI] [PubMed] [Google Scholar]
- Sandvig K., Torgersen M. L., Raa H. A. and VAN Deurs B. (2008). Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem. Cell Biol. 129, 267-276. 10.1007/s00418-007-0376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G., Weiner O. D., Neptune E. R., Sedat J. W. and Bourne H. R. (1999). Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol. Biol. Cell 10, 1163-1178. 10.1091/mbc.10.4.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Milne L. and Hawkins P. (2008). Moving towards a better understanding of chemotaxis. Curr. Biol. 18, R485-R494. 10.1016/j.cub.2008.04.048 [DOI] [PubMed] [Google Scholar]
- Subramanian B. C., Majumdar R. and Parent C. A. (2017). The role of the LTB4-BLT1 axis in chemotactic gradient sensing and directed leukocyte migration. Semin. Immunol. 33, 16-29. 10.1016/j.smim.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova E. S., Gripentrog J. M. and Miettinen H. M. (2005). Different endocytosis pathways of the C5a receptor and the N-formyl peptide receptor. Traffic 6, 100-115. 10.1111/j.1600-0854.2004.00256.x [DOI] [PubMed] [Google Scholar]
- Sanjana N. E., Shalem O. and Zhang F. (2014). Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783-784. 10.1038/nmeth.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieblemont N., Wright H. L., Edwards S. W. and Witko-Sarsat V. (2016). Human neutrophils in auto-immunity. Semin. Immunol. 28, 159-173. 10.1016/j.smim.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Tucker K. A., Lilly M. B., Heck L. J. and Rado T. A. (1987). Characterization of a new human diploid myeloid leukemia cell line (PLB-985) with granulocytic and monocytic differentiating capacity. Blood 70, 372-378. [PubMed] [Google Scholar]
- Tuma P. L., Nyasae L. K., Backer J. M. and Hubbard A. L. (2001). Vps34p differentially regulates endocytosis from the apical and basolateral domains in polarized hepatic cells. J. Cell Biol. 154, 1197-1208. 10.1083/jcb.200105138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas P., Barbier L., Sáez P. J. and Piel M. (2017). Mechanisms for fast cell migration in complex environments. Curr. Opin. Cell Biol. 48, 72-78. 10.1016/j.ceb.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Vines C. M., Revankar C. M., Maestas D. C., Larusch L. L., Cimino D. F., Kohout T. A., Lefkowitz R. J. and Prossnitz E. R. (2003). N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J. Biol. Chem. 278, 41581-41584. 10.1074/jbc.C300291200 [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F. and Zallen J. A. (2009). Junctional trafficking and epithelial morphogenesis. Curr. Opin. Genet. Dev. 19, 350-356. 10.1016/j.gde.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Vines C. M., Buranda T., Cimino D. F., Bennett T. A. and Prossnitz E. R. (2004). N-formyl peptide receptors cluster in an active raft-associated state prior to phosphorylation. J. Biol. Chem. 279, 45175-45184. 10.1074/jbc.M407053200 [DOI] [PubMed] [Google Scholar]
- Yang H. W., Collins S. R. and Meyer T. (2016). Locally excitable Cdc42 signals steer cells during chemotaxis. Nat. Cell Biol. 18, 191-201. 10.1038/ncb3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.