Fig. 6.
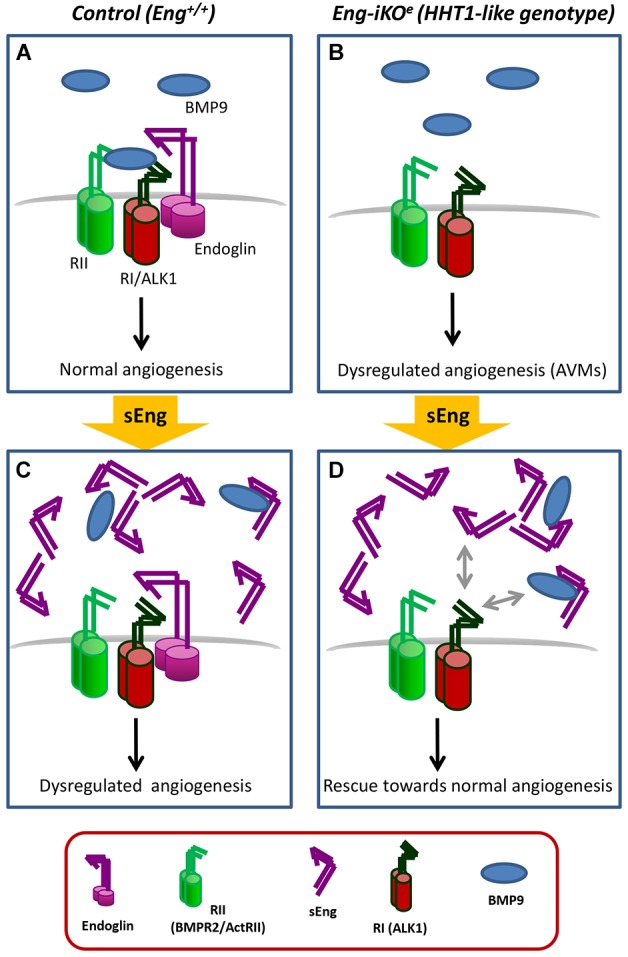
Hypothetical model of sEng action on the vasculature. (A,B) In normal endothelial cells, membrane endoglin is a component of a receptor complex that contains type I (RI; ALK1), and type II (RII; BMPR2/ActRII) TGF-β receptors, which can be activated by BMP9, leading to an equilibrium between pro- and antiangiogenic factors (A). In endoglin-silenced endothelial cells (Eng-iKOe), BMP9 cannot bind to endoglin and BMP9-dependent signaling is disturbed, leading to a dysregulated expression of angiogenic factors, decreased migration during angiogenesis and the presence of AVMs (B). (C,D) In normal endothelial cells, the circulating extracellular region of endoglin (sEng) can interact with BMP9, sequestering the ligand, interfering with the intracellular signaling of the receptor complex and changing the angiogenesis balance towards a dysregulated state (C). In Eng-iKOe, BMP9 cannot bind to membrane endoglin, but interacts with sEng, and the resulting BMP9/sEng complex interacts with the ALK1 receptor on the cell membrane, enhancing the proangiogenic ALK1 signaling and decreasing the incidence of AVMs (D). The involvement of endoglin in the TGF-β1/ALK5 signaling pathway of endothelial cells has been omitted for simplification.
