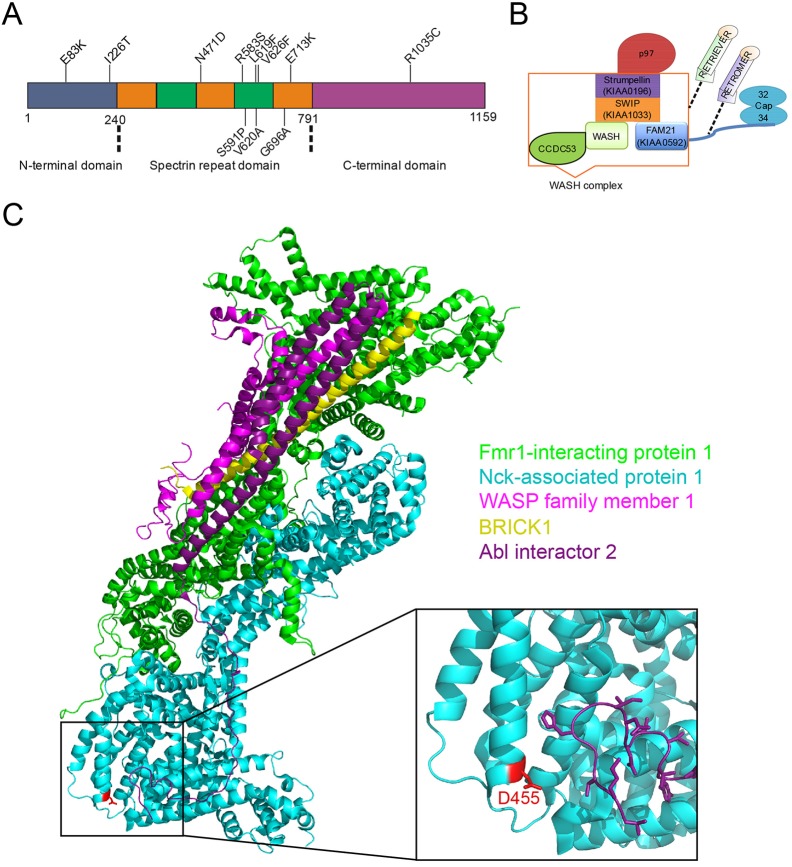
Fig. 1.
Schemes of strumpellin and the WASH complex, and three-dimensional structure of the WAVE complex. (A) Domain structure of strumpellin. Strumpellin is composed of an N-terminal domain, a middle domain with five spectrin-like repeats and a C-terminal domain. The amino acids of the SPG8-associated point mutations are shown at their approximate positions. (B) Model of the WASH complex and its associated complexes and proteins. The five core proteins – WASH, FAM21 (KIAA0592), CCDC53, SWIP (KIAA1033) and strumpellin (KIAA0196) – of the WASH complex, as well as two of the known interacting proteins, Cap32/34 and p97, are depicted. For completeness, the retromer and retriever complexes, which very likely interact with the WASH complex in a mutually exclusive manner, are also shown. (C) Representation of the WAVE complex (PDB 3p8c), showing the different components of the complex in different colours. The hetero-pentameric WAVE regulatory complex is composed of Fmr1-interacting protein 1 (also known as Sra1, green), Nck-associated protein 1 (Nap1, turquoise), WASP family member 1 [WASF1 (also known as WAVE1), pink], Abl interactor 2 (Abl2, purple) and HSPC300 (BRICK1, yellow). These proteins correspond to SWIP, strumpellin, WASH, FAM21 and CCDC53, respectively, in the WASH complex. Nap1 D455 is depicted in red. Figure prepared with PyMOL (DeLano, 2002) and Inkscape (https://inkscape.org/en/).