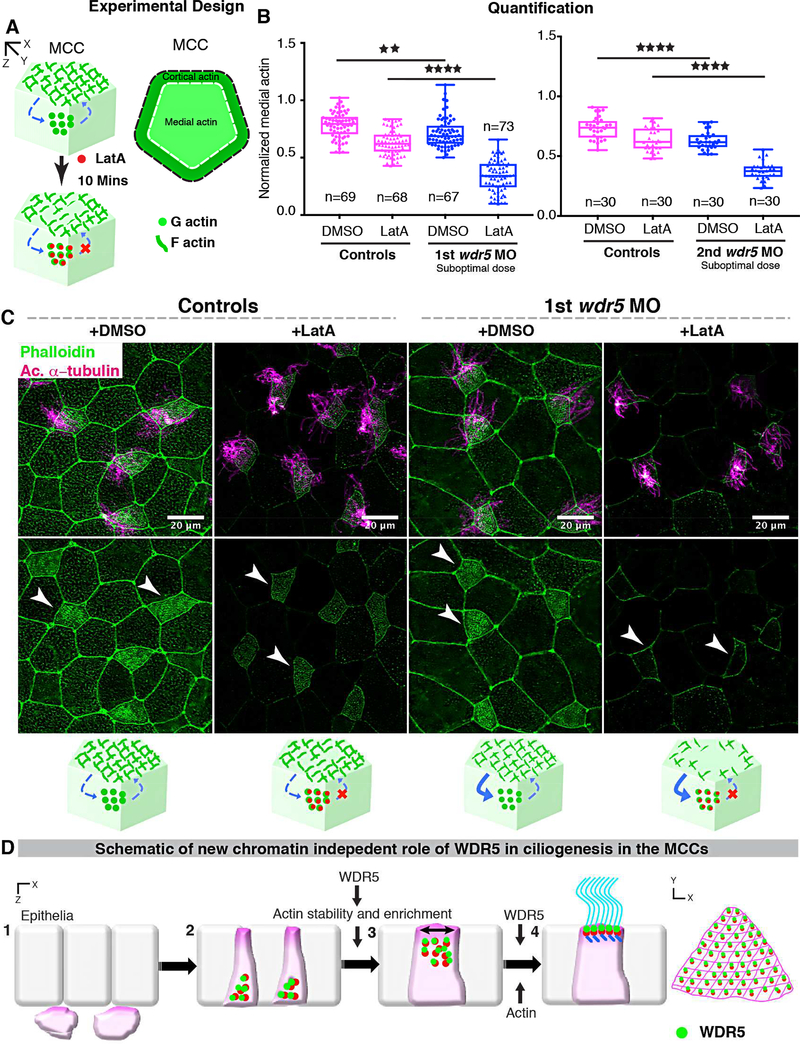
Figure 7: Wdr5 is essential for stabilization of F-actin.
(A) Schematic showing the experimental design to examine the rate of F-actin disassembly in MCCs of Xenopus embryos. To isolate the effect of WDR5 in disassembly, we exposed epidermal MCCs to Latrunculin A (LatA) treatment for 10 mins. Latrunculin A specifically binds to G-actin in stoichiometric 1:1 ratio preventing the F-actin polymerization. Thus, exposure to LatA prevents the assembly but not disassembly of F-actin allowing us to evaluate the effect of WDR5 depletion on F-actin disassembly in MCCs. Based on earlier results that medial actin is necessary for apical expansion and our results that Wdr5 depletion leads to loss of medial Factin enrichment, we specifically examined the medial F-actin intensity in controls and wdr5 morphants. Medial actin intensity was normalized to cortical actin to allow us to combine results from different experiments for statistical comparison.
(B) Quantification of normalized medial actin intensity in the MCCs of controls and suboptimal dose of wdr5 morphants after exposure to DMSO (vehicle) or LatA (2 μM) for 10 mins using two non-overlapping wdr5 MOs. Our results show dramatic reduction in actin intensity in response to LatA in wdr5 morphants compared to DMSO only. n = number of MCCs.
(C) Immunofluorescence showing Xenopus epidermal MCCs labeled for F-actin (Phalloidin) and cilia (anti-acetylated α-tubulin) in controls and wdr5 morphants after exposure to DMSO (vehicle) or LatA (2uM) for 10 mins. Cartoon depicts the hypothesized rates of disassembly and the effect of LatA on medial actin enrichment in controls and wdr5 morphants.
(D) Schematic of a model proposing the role of WDR5 in formation of a MCC. WDR5 binds to basal bodies as basal bodies are synthesized deep in the cytoplasm. WDR5 then migrates apically, where F-actin organizes around WDR5. WDR5 interacts with F-actin to stabilize actin network essential for apical expansion and basal body distribution. In the final stages, WDR5 anchors basal bodies to apical actin network to form functional MCCs.