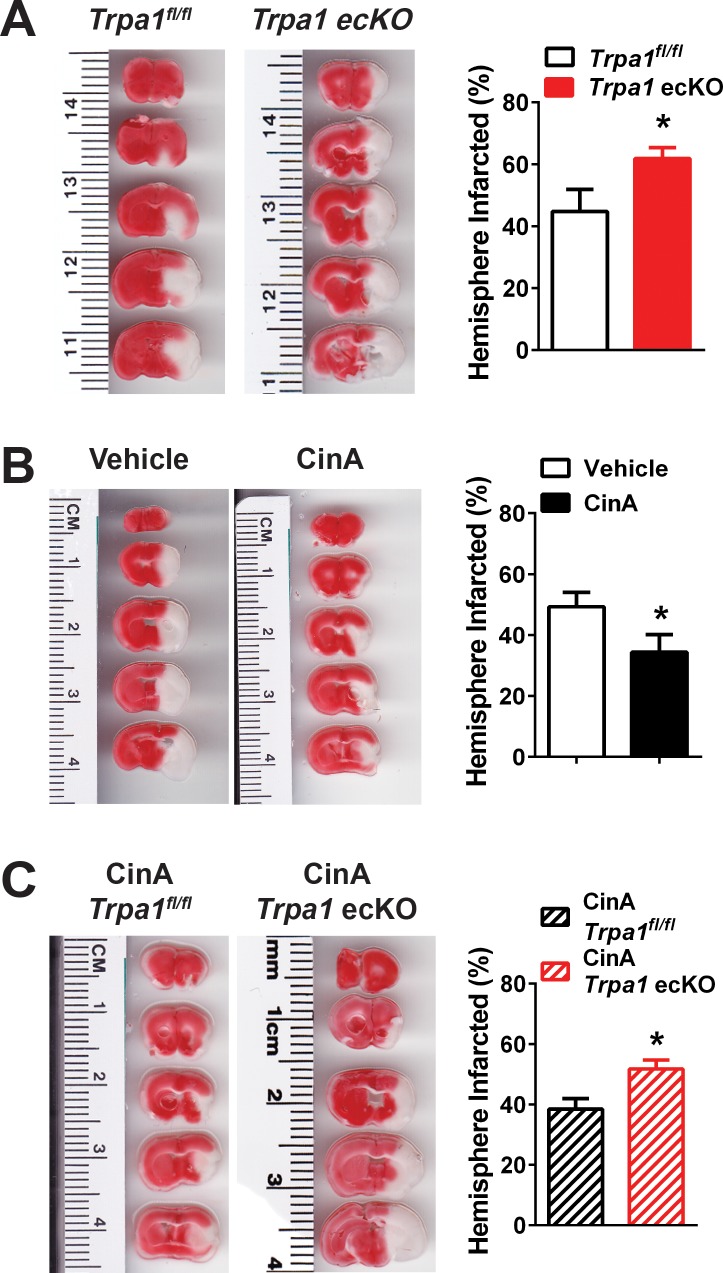
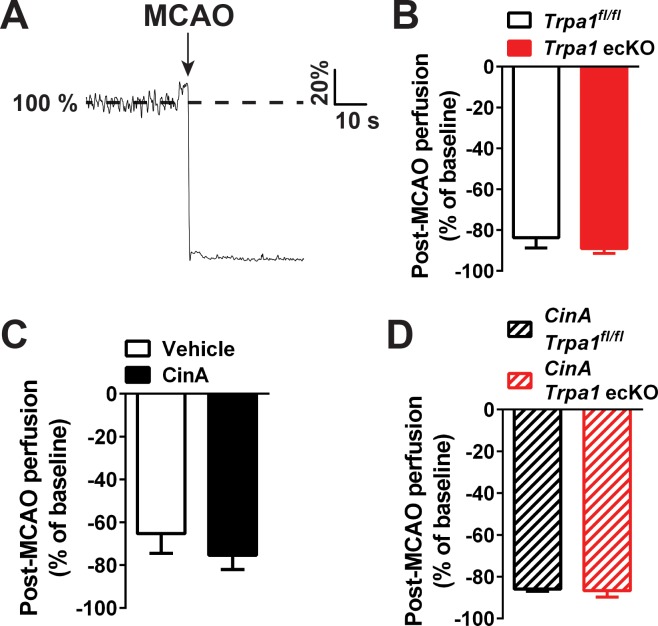
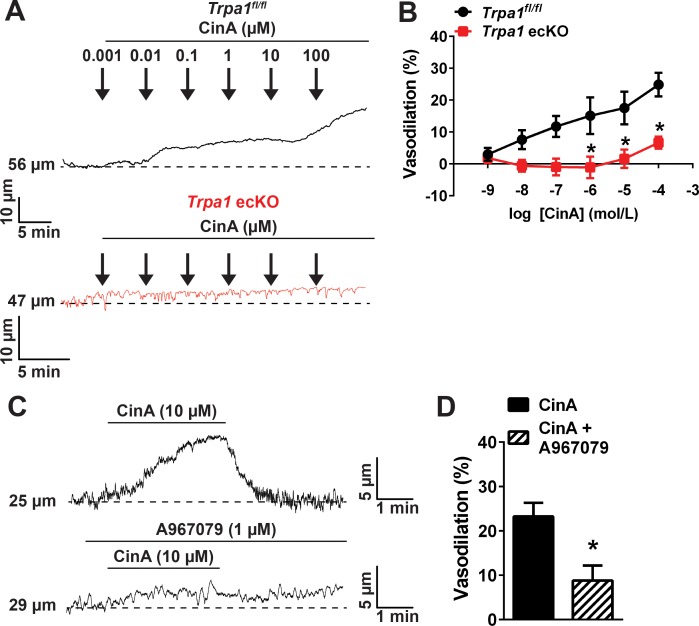
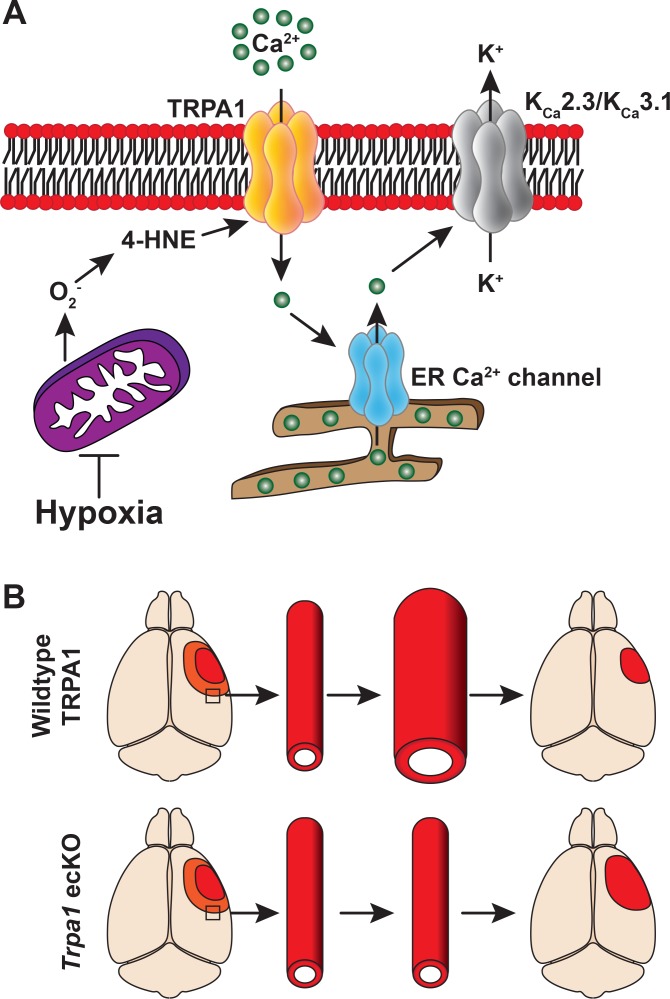
Figure 8. Endothelial cell TRPA1 channel activity protects against ischemic strokes.
(A) Representative photographs of brain slices (left) and summary data (right) showing significantly greater ischemic damage 24 hr after MCAO in Trpa1 ecKO mice compared with Trpa1fl/fl. Brain slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC), which stains metabolically active tissue red, whereas infarcted tissue remains unstained (white). Infarcted areas were quantified and expressed as a percentage of total hemisphere area (*p<0.05, Student’s t-test; n = 5–5 mice). (B) Representative photographs of brain slices (left) and summary data (right) showing reduced cerebral ischemic damage in wildtype C57/bl6 mice treated with the TRPA1 channel activator cinnamaldehyde (CinA, 50 mg/kg i.p.), injected 15 min after MCAO (*p<0.05 for CinA vs. vehicle, Student’s t-test; n = 5–6 mice). (C) Representative photographs of brain slices (left) and summary data (right) showing that the protective effects of CinA were blunted in Trpa1 ecKO mice (*p<0.05 for CinA-treated Trpa1 ecKO mice vs. CinA-treated Trpa1fl/fl mice, Student’s t-test; n = 6–5 mice). Legends for Supplemental Figures.