Figure 1. Structural features of hnRNP A1.
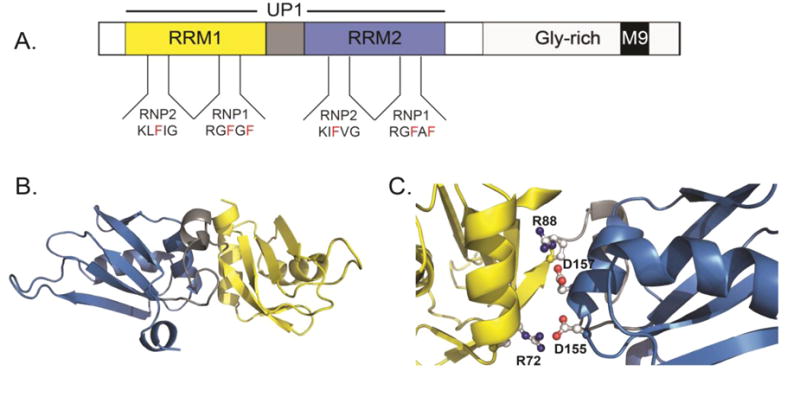
(A) The domain organization of full-length hnRNP A1 showing the N-terminal RNA binding domains (RRM1=yellow, Inter-RRM linker=gray, and RRM2=blue) and the C-terminal domain (light gray) with M9 nuclear localization signal depicted as a black box. The tandem RRMs of hnRNP A1 collectively make up the UP1 protein, residues 1-196. The RNP1 and RNP2 submotifs are also depicted for each RRM. (B) The solution NMR structure (2LYV) of UP1 color-coded as in part A. (C) A zoomed view of the alpha helical side of UP1 showing the conserved salt bridge interactions that stabilize the relative orientation of RRM1 and RRM2.
