Abstract
Chronic exposure to inorganic arsenic creates various health problems. Ixora coccinea flower extract was investigated for its ability to protect against arsenic-induced cytotoxicity and genotoxicity in CHO cell line. MTT assay confirmed the efficacy of the extract in ameliorating arsenic-induced cytotoxicity. The value (48 mM) of 24 h inhibitory concentration (IC50) of sodium arsenate for CHO cells was obtained by MTT assay. Various free radical scavenging assays like DPPH, ABTS and nitric oxide scavenging assay confirmed antioxidant activity of the Ixora coccinea flower extract. Pretreatment of the extract significantly inhibited the arsenic-induced DNA damage (p < 0.01) in CHO cells. The extract administration significantly (p < 0.01) inhibited the intracellular ROS and depolarization of mitochondrial membrane induced by sodium arsenate. Ixora coccinea flower extract reduced oxidative stress in cells. Antioxidant enzymes like catalase and SOD activity was restored significantly (p < 0.01) in pretreated CHO cells. Ixora coccinea flower extract also exhibited the anti-apoptotic potential by decreasing the percentage apoptotic index (p < 0.01). These results may expand the applications of Ixora coccinea flowers as an alternative food with antioxidant properties and protective functions against arsenic (iAs) induced toxicological effects.
Keywords: Ixora coccinea, CHO cells, ROS, Cytotoxicity, Comet assay
Introduction
Arsenic is a metalloid ubiquitously present in the environment. Drinking arsenic contaminated water is the major source of human exposure to arsenic (Wang et al. 2011). According to WHO the permissible limit is 50 µg/l. Chronic exposure to high concentrations of inorganic arsenic leads to deleterious effects in living cells (Mazumder 2008). Arsenic induces cytotoxicity through the generation of intracellular ROS and oxidative stress which results in the altered enzyme activities (Aung et al. 2013). Increased ROS production causes oxidative damage to DNA and apoptosis in cells. Arsenate also leads to lipid peroxidation in exposed cells (Singh et al. 2011).
Ixora coccinea has been used traditionally in folk medicine (John 1984) and in Ayurveda to treat several diseases. It is called as Bandhuka, Bandhujivaka, Ishwara, Parali, Paranti, Raktaka in Sanskrit, Kangan, Rajana, Rangan, Rookmini, Rugmini in Hindi, Rookmini, Kangan, Kannada-Gudde dosal, Kepala, Kisukare, Kempulagida in Hindi, Patkali, Podkali, Malayalam-Chethi, Thechi, Thetti in Konkani, Bakali, Bakora, Pankul, Patkalin, Pendgul, Oriya-Bondhuko, Romoniphulo in Marathi, Koran kullai, Sedaram, Sinduram rangan Vetchi in Tamil, Bandhujivakamu, Bandhujivamu, Bandhukamu, mankana, manmadabanam in Telugu (Baliga and Kurian 2012). In Ayurveda, flowers are used to treat diseases such as dysentery, leucorrhoea, dysmenorrhoea, hypertension, menstrual problems, sprains, bronchitis fever, chronic ulcers, sores, skin diseases and scabies (Saha et al. 2008; Sankaranarayanan et al. 2010). The flowers are used as a wound healing agent, to treat catarrhal bronchitis as well as dysentery (Sivarajan et al. 1994). Studies indicate that the Ixora coccinea has antioxidant and antibacterial properties. The shade dried, powdered flowers are used in treating eczema (Sivaperumal et al. 2009). The concoction prepared from the root is traditionally used in treating nausea, anorexia and hiccups. Sores and chronic ulcers are also treated with the use of finely pulverized dry roots (Vadivu et al. 2010). Ixora coccinea flowers and fruits are edible and used as functional food (Baliga and Kurian 2012). In the southern part of India they are used in different cuisines (raw) as a nutrient supplement.
In this paper, the interactions of CHO cells with sodium arsenate and the reversal of its toxic effects by Ixora coccinea aqueous extract has been studied by different assays including cell survival assay, ROS assay, comet assay, MMP and apoptosis. Ixora coccinea aqueous extract was evaluated for the ability to attenuate arsenic-induced cytotoxicity and genotoxicity in CHO cells. Phytochemical studies were performed to estimate the total phenols, total flavonoids. Antioxidant ability of aqueous extract was evaluated through DPPH, ABTS, Nitric oxide scavenging assay and total antioxidant capacity. MTT assay was carried out in CHO cells to evaluate cell viability. Altered Intracellular ROS, mitochondrial membrane potential leading to change in florescent intensity were read on FACS. The extent of DNA damage was estimated by comet assay in an alkaline medium. Cellular apoptosis was evaluated in CHO cells. Antioxidant enzymes like SOD and catalase levels were also estimated.
Materials and methods
Plant material
The plant was authenticated by Botanist, Professor and Head (Ret.) Aravinda Hebbar, A voucher specimen (PP 619) has been deposited in the herbarium of our University, Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, MAHE, and Manipal for future reference.
Preparation of samples
Shade dried Ixora coccinea flower powder was soaked in distilled water with 1% chloroform for 7 days. The extract thus obtained was filtered to remove fiber, then concentrated with controlled temperature in a rotary evaporator. The powder thus obtained was dissolved in distilled water to desired concentrations for experimental purpose.
Determinations of total phenols
Total phenols were measured in aqueous extract of Ixora coccinea with the Folin–Ciocalteau reagent. The total concentrations of phenolics in the extracts were measured using gallic acid as standard. 1 ml of extract or standard solution was mixed thoroughly with 5 ml of FC reagent with ten times dilution in water and 4 ml of 700 mM sodium carbonate, incubated for 2 h and then absorbance was recorded at 765 nm using UV-spectrophotometer (Slinkard and Singleton 1977). The total content of phenolic compound expressed as gallic acid equivalents mg/g of flower extract.
Determinations of total flavonoid content
Total flavonoids in Ixora coccinea aqueous extract were measured with the aluminium chloride colorimetric method. Quercetin was kept as standard, 500 µl of standard or extract was mixed with methanol (1.5 ml), aluminium chloride (0.1 ml, 10%), potassium acetate (0.1 ml, 1M) and distilled water (2.8 ml), after 30 min absorbance was recorded at 415 nm with the help of UV-spectrophotometer (Chang et al. 2002). The total flavonoid content was represented as quercetin equivalents mg/g of the dry flower extract.
DPPH free radical scavenging assay
The free radical scavenging activity of the aqueous extract of Ixora coccinea was measured in the presence of DPPH. 1 ml of DPPH (0.1 mM) solution in methanol was added to 1.0 ml of extract with various concentrations, after 20 min absorbance was recorded at 517 nm using spectrophotometer (Bansal et al. 2011).
ABTS free radical scavenging assay
ABTS free radical assay was performed using 7 mM ABTS solution with potassium persulphate (2.4 mM), mixed and incubated for 15 h in the dark at room temperature. Various concentrations of extract were prepared in methanol and 20 ml of test solutions were mixed with 180 ml of ABTS free radical solution, incubated for 20 min and absorption was measured at 750 nm (Tachakittirungrod et al. 2007).
Nitric oxide scavenging assay
Nitric oxide scavenging assay was performed using the Griess method. 2 ml of Sodium nitroprusside (10 mM) was mixed with 0.5 ml of PBS, 0.5 ml of standard or extract solutions at various concentrations and incubated at 25 °C for 1 h 30 min. 0.5 ml of the reaction mixture was mixed with 1 ml sulphanilic acid reagent (0.33% sulphanilic acid in 20% glacial acetic acid) and allowed to stand for 5 min, followed by mixing of 1 ml 0.1% naphthyl ethylene diamine dihydrochloride. After 30 min absorbance was recorded at 540 nm (Gouthamchandra et al. 2010).
Total antioxidant capacity
The phosphomolybdenum method was used to measure the total antioxidant capacity of the extract. 0.1 ml of standard/extract solution was mixed with 0.3 ml of reagent (4 mM ammonium molybdate, 0.6 M sulfuric acid with 28 mM sodium phosphate) and kept for 90 min at 95 °C. The mixture was allowed to attain room temperature, then absorbance was measured at 695 nm. Ascorbic acid was used to obtain the calibration curve, and represented as ascorbic acid equivalents.
Cell culture
CHO cells were purchased from National Centre for Cell Sciences (Pune), India. The cells were cultured in T-25 culture flask (Falcon, Becton Dickinson, USA), containing DMEM supplied with 10% FBS, 1% penicillin–streptomycin, at 37 °C in a CO2 incubator (NuAire, Plymouth, MN, USA) with 5% CO2 in 95% air. For different assays, cells were maintained at 80–85% confluency in culture flasks and used. Trypan blue dye exclusion method was used to check the viability of cells.
MTT assay
Cytotoxicity of the arsenate, was evaluated using the MTT assay. 80–90% confluent cells were trypsinized and seeded at a density of 1 × 104 cells per well and treated with sodium arsenate, Ixora coccinea extract alone and arsenate in combination with Ixora coccinea extract to evaluate the cytotoxicity. 0.1 ml of DMSO was added at the end to dissolve formazan crystals, and absorbance was recorded at 550 nm (Mosmann et al. 1983).
Determination of reactive oxygen species
To measure the intracellular ROS production, cells (seeded at a density of 3 × 105) were treated with arsenate, Ixora coccinea extract and combination of extract as well as arsenate with fresh medium and the cultures were incubated for 1 h (Bai and Cederbaum 2003). After the incubation, the medium was removed and supplemented with 5 µM DCFDA for 30 min at 37 °C in 5% CO2 incubator. Cells were trypsinized and suspended in PBS, analyzed using flow cytometer (Becton Dickinson, USA).
Estimation of mitochondrial membrane potential (∆Ψm)
To measure the mitochondrial membrane potential cells were seeded at a density of 3 × 105 in 6 cm2 Petri dishes. Cells were incubated for 24 h with arsenate, Ixora coccinea flower extract and in a combination of arsenate with extract, followed by the addition of Rhodamine 123 (5 µg/ml) and incubated at 37 °C for 30 min in 5% CO2. Cells were trypsinised, collected, washed twice with PBS and centrifuged at 1000 rpm for 10 min (Scaduto and Grotyohann 1999). Cells were finally suspended in 1 ml of cold PBS and samples were read on FACS Calibur (Becton Dickinson, USA).
Single cell gel electrophoresis (SCGE)
Single strand DNA breaks were quantified by comet assay in an alkaline medium. 7 × 105 cells were seeded in 6 cm2 petri plates. Cells were exposed to arsenate, Ixora coccinea and in combination kept for 24 h, later cells were trypsinized and collected in PBS. Agarose covered slides were layered with suspended cells again recoated with agarose. The slides were dipped in the lysing solution maintained at 4 °C overnight, lysed cells were subjected to electrophoresis for 28 min (300 mA, 20V). The slides were washed with neutralizing buffer. Ethidium bromide stained slides were immediately observed under the fluorescence microscope and photographed with coomat, analyzed with software (Singh et al. 1988).
AO/EtBr dual staining
3 × 105 cells were seeded per well in a 6 well plate overnight and kept in an incubator at 37 °C, in 5% CO2, then treated with arsenate, Ixora coccinea and in combination. Later 300 µl of AO/EtBr (30/20 µg/ml) was added to each well then the plate was kept at 37 °C for 30 min. Finally washed with PBS and visualized under the fluorescence microscope for nuclear fragmentation and condensation (Renvoize et al. 1998).
Catalase activity estimation
3 × 105 cells were seeded per well in a 6 well plate overnight kept in an incubator at 37 °C, in 5% CO2, then treated with arsenate, Ixora coccinea and in combination kept for 24 h, later cells were trypsinized and collected in PBS. The catalase assay was performed as described by (Aebi 1974). The phosphate buffer (prepared by mixing 50 mM KH2PO4 and 50 mM Na2HPO4 in a 1:1.5 v/v ratio) having pH 7.0 was mixed with 30 mM H2O2 to get an absorbance value of around 0.5 using a spectrophotometer. This reading was taken as the blank. If the value was higher than 0.5 then the amount of buffer in the reaction mixture was increased and if it showed a lower value than the H2O2 content was increased. The volume of the buffer and H2O2 mixture was kept constant at 0.9 ml throughout the experiment. 50 µl of sample was added to the cuvette containing the reaction mixture and the decrease in absorbance per unit time was measured for each of the samples. The decomposition of H2O2 can be followed directly by the decrease in absorbance at 240 nm. The difference in absorbance (A240) per unit time is a measure of catalytic activity.
Estimation of SOD activity
3 × 105 cells were seeded per well in a 6 well plate overnight kept in an incubator at 37 °C, in 5% CO2, then treated with arsenate, Ixora coccinea and in a combination of arsenate with extract kept for 24 h, later cells were trypsinized and collected in PBS. 1850 µl of sodium carbonate buffer was mixed with 50 µl of the sample. Further 100 µl of epinephrine was added to the reaction mixture and the increase in the absorbance which is proportional to the rate of autoxidation of epinephrine to adrenochrome was read immediately at 480 nm using a spectrophotometer.
Results and discussion
The role of natural products and folk medicine in the amelioration of the toxic effects of arsenic is scanty (Bhattacharya 2017). Ixora coccinea extract was prepared and analyzed for phytochemical constituents and its potential to ameliorate arsenic-induced cytotoxicity in CHO cells. The phytochemical evaluation of the aqueous extract showed antioxidant activities providing the mechanistic answer to the observed amelioration of arsenic-induced toxicity in cells.
Phenols are known as reducing agents, free radical scavengers and metal chelators. They are an important class of antioxidants because of their chemical structure. There are reports of flavonoids being scavengers of free radicals and has antioxidant properties. Phenol compounds and flavonoids were detected in Ixora coccinea flower aqueous extract.
Reactive nitrogen species have a role to play in metal induced toxicity. In spite of the defense system existing in cells, an imbalance in redox status leads to oxidative stress and DNA damage. Ixora coccinea aqueous extract possesses the nitric oxide scavenging capacity indicating its quenching ability of free radical. ABTS scavenging indicates that the presence of phenol-related compounds is responsible for ABTS free radical scavenging activity. The total antioxidant activity of aqueous flower extract was measured using the phosphomolybdenum complex.
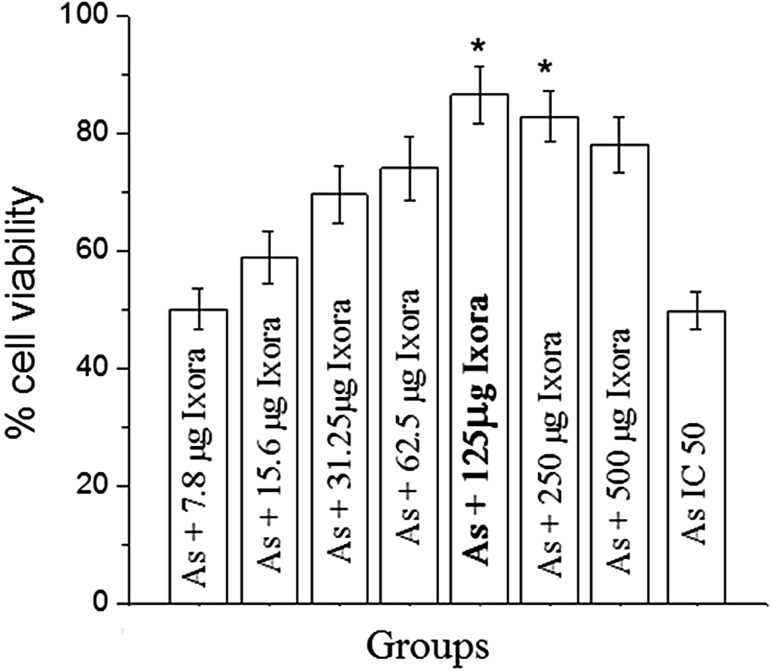
CHO cells were exposed to different concentrations of sodium arsenate for 24 h to obtain the inhibitory concentration (IC50) i.e., 48 µM. Various concentrations of Ixora coccinea flower aqueous extract did not have any adverse effect on cell viability when treated for 24 h. Increase in cell viability was observed when the cells were pretreated with Ixora coccinea extract for about 2 h before the exposure to sodium arsenate (IC50). Maximum survival was observed in 125 µg extract and was significant (p < 0.01) when compared to other concentrations of the extract (Fig. 1).
Fig. 1.
Alterations in cell viability of CHO cells treated with different concentrations of Ixora coccinea flower aqueous extract and Arsenate (iAs) using MTT assay
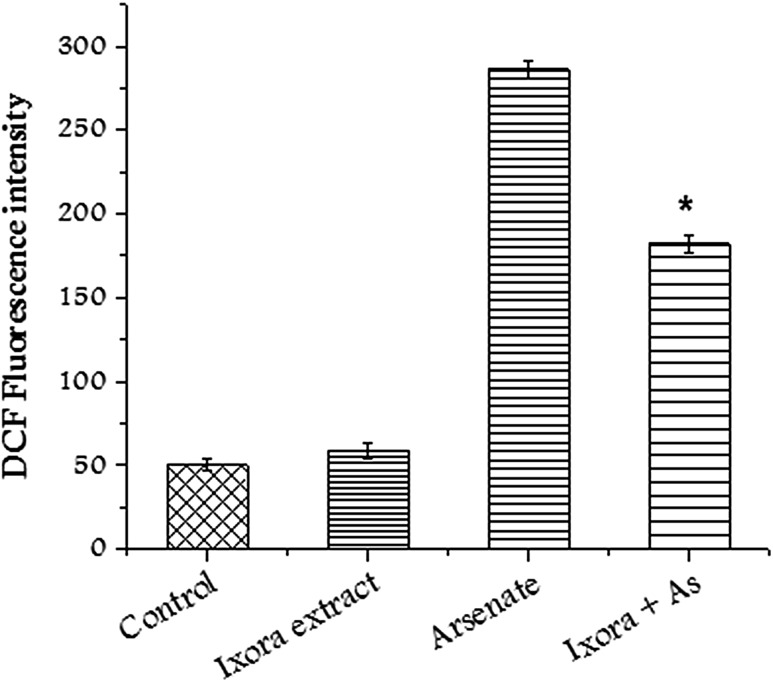
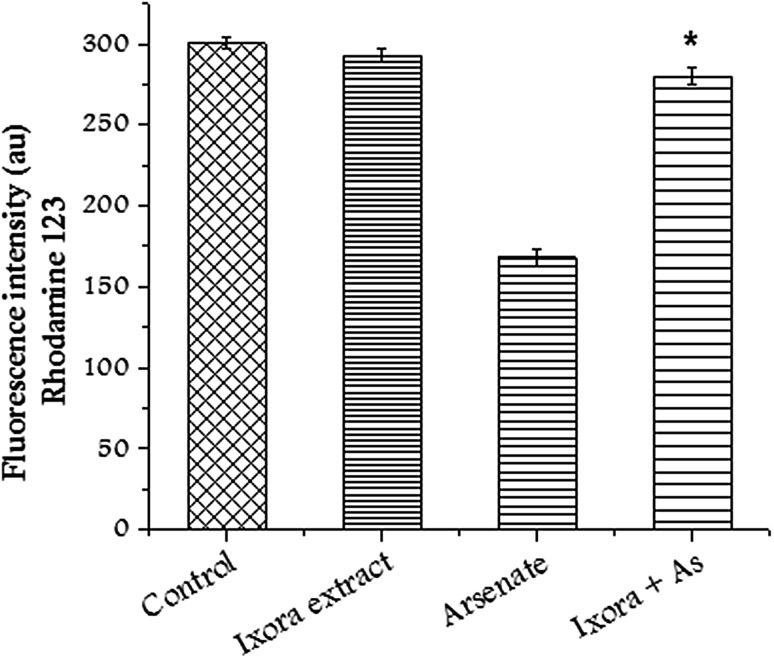
ROS concentration was measured using DCFHDA (2′, 7′-dicholorofluorescein diacetate). High concentration of ROS induces lipid peroxidation and oxidative stress. However, the pretreatment of cells with Ixora coccinea extract significantly reduced the concentration of ROS in CHO cells in the presence of sodium arsenate (Fig. 2). Transmembrane potential dissipation increases with the increase in mitochondrial damage. CHO cells treated with sodium arsenate showed alteration in membrane potential. Pretreatment of CHO cells with Ixora coccinea extract restored the membrane potential and change was significant (p < 0.01) compared to sodium arsenate treated cells (Fig. 3).
Fig. 2.
Alterations in intracellular ROS after treating CHO cells with Ixora coccinea extract (125 µg/mL), Arsenate (IC50) and in combination. Significant (p < 0.01) alteration observed in treated group with respect to arsenate
Fig. 3.
Alteration in mitochondrial membrane potential after treating CHO cells with Ixora coccinea extract (125 µg/mL, arsenate and in combination. Significant (p < 0.01) alteration observed in treated group with respect to arsenate
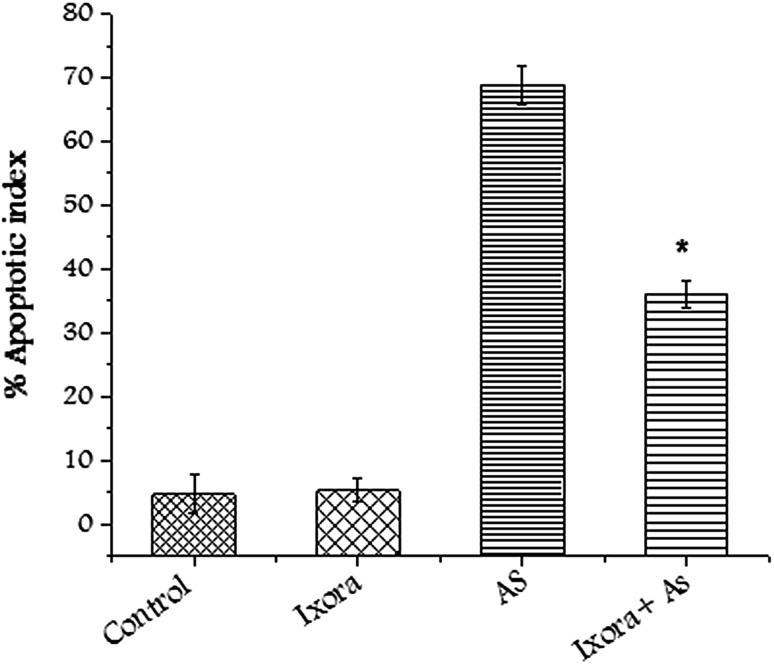
Comet assay (alkaline) was used to measure the DNA damage induced by sodium arsenate. Sodium arsenate induced single strand breaks in CHO cells when exposed to its inhibitory concentration. Pretreating of CHO cells with Ixora coccinea flower extract before treatment of sodium arsenate showed significant (p < 0.01) decrease in DNA damage (Fig. 4). Apoptotic execution is followed by chromatin condensation and morphological changes in cells. Apoptotic index was calculated counting random 100 cells (Fig. 5). Sodium arsenate induced apoptosis was reduced significantly (p < 0.01) after the CHO cells were pretreated with Ixora coccinea extract.
Fig. 4.
Altered DNA damage after treating CHO cells with Ixora coccinea extract (125 µg/mL), arsenate(IC50) and in combination. Significant (p < 0.01) alteration observed in treated group with respect to arsenate
Fig. 5.
Graph showing altered apoptotic index after treating CHO cells with Ixora coccinea extract (125 µg/mL), arsenate (IC50) and in combination. Significant (p < 0.01) alteration observed in treated group with respect to arsenate
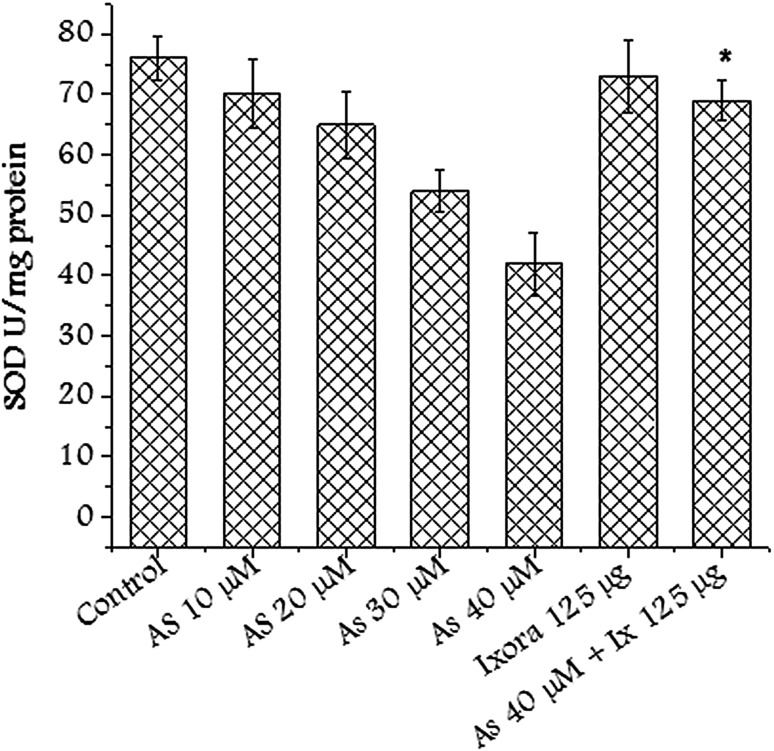
Superoxide dismutase is thought to be present in all aerobic cells wherein it has a crucial function of protecting living organisms against unrestrained reactivity of the superoxide radical (O2−) which is generated as a result of the univalent reduction of oxygen either chemically or enzymatically. SOD enzyme level was restored in the group pretreated with Ixora coccinea extract (Fig. 6). This could be attributed to free radical scavenging activity of the extract (Table 1).
Fig. 6.
SOD activity after treating CHO cells with Ixora coccinea extract (125 µg/mL), arsenate (various concentrations) and in combination. Significant (p < 0.01) alteration observed in treated group with respect to arsenate
Table 1.
Free radical scavenging and antioxidant capacity of the Ixora coccinea aqueous extract
| Total phenols (mg GAE/g of flower extract) | 42.21 + 0.24 |
| Total flavonoids (mg QE/g of flower extract) | 2.4 + 0.004 |
| DPPH radical scavenging activity | 98.42 + 3.2 |
| ABTS radical scavenging activity | 81.10 + 1.8 |
| Nitric oxide scavenging assay | 118.26 + 8.2 |
| Total antioxidant capacity | 480 + 1.34 |
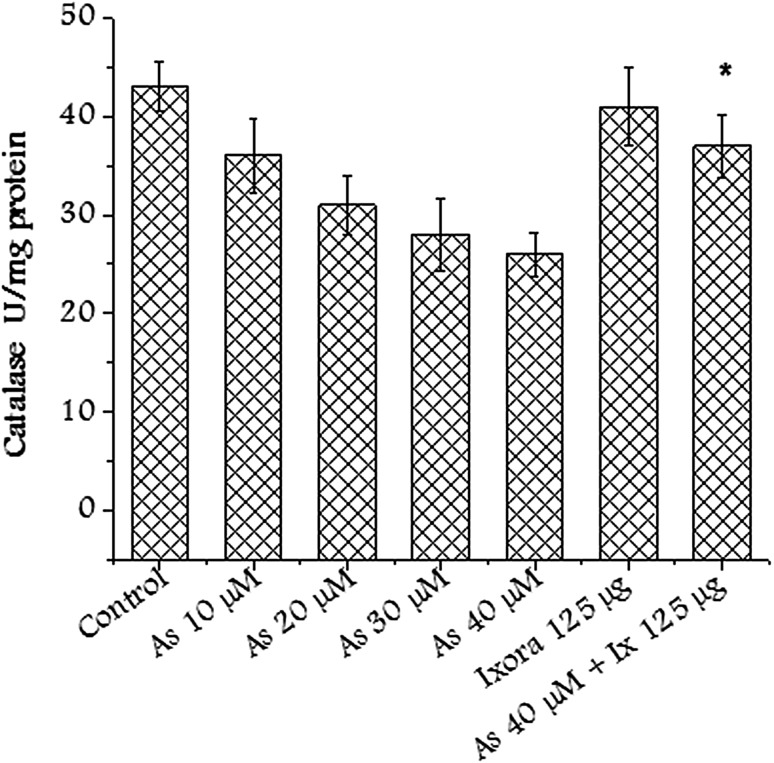
Catalase is an antioxidant enzyme ubiquitously present in mammalian and non-mammalian aerobic cells containing a cytochrome system. Catalase enzyme level was restored in the group pretreated with Ixora coccinea extract (Fig. 7). The antioxidant behavior of the extract helped in restoring the enzyme activity.
Fig. 7.
Catalase activity after treating CHO cells with Ixora coccinea extract (125 µg/mL), arsenate (various concentrations) and in combination. Significant (p < 0.01) alteration observed in treated group with respect to arsenate
Dietary supplementation or intervention of naturally occurring nutrients is useful in preventing arsenic-induced toxicological effects and oxidative damage to the cells. A number of edible plants has been listed to reverse the effect of arsenic-induced toxicity (Baliga and Kurian 2012). Ixora coccinea flower extract possesses ameliorative properties because of the presence of phenolics, flavonoids and rutin like known antioxidants. Ixora coccinea is used in traditional medicine and there are limited studies concerning metal toxicity or metalloid toxicity.
Conclusion
The present study is focused on amelioration of arsenic-induced cytotoxicity and genotoxicity by Ixora coccinea a known functional food. The protective effect of Ixora coccinea could be ascribed to its antioxidant properties of flavonoid and phenolic components of the extract. Aqueous extract of Ixora coccinea flower attenuated arsenic-induced oxidative stress, cytotoxicity and genotoxicity in CHO cells. Pretreatment of the extract also restored the activity of antioxidant enzymes like SOD and catalase to near normal levels reducing the oxidative stress. The experimental results indicate the protective role of Ixora coccinea against arsenic (iAs) induced toxicological effects. The current study demonstrated the cytoprotective potential of the extract, which may be attributed to quenching of the ROS generated in the CHO cells due to oxidative stress induced by sodium arsenate. Ixora coccinea flowers can be used as functional food in the affected regions to reduce the harmful effects of the arsenic due to the consumption of arsenic contaminated drinking water.
Acknowledgements
The authors are grateful to Dr. Satish Rao, Professor of Radiobiology division, School of Life Sciences, Manipal Institute of Technology, Manipal College of Pharmaceutical Sciences Manipal Academy of Higher Education for providing laboratory facilities.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 2. Weinheim: Verlag Chemie; 1974. pp. 673–684. [Google Scholar]
- Aung KH, Kurihara R, Nakashima S, Maekawa F, Nohara K, Kobayashi T, Tsukahara S. Inhibition of neurite outgrowth and alteration of cytoskeletal gene expression by sodium arsenite. Neurotoxicology. 2013;34:226–235. doi: 10.1016/j.neuro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Bai J, Cederbaum AI. Catalase protects HepG2 cells from apoptosis induced by DNA-damaging agents by accelerating the degradation of p53. J Biol Chem. 2003;278:4660–4667. doi: 10.1074/jbc.M206273200. [DOI] [PubMed] [Google Scholar]
- Baliga MS, Kurian PJ. Ixora coccinea Linn.: Traditional uses, phytochemistry and pharmacology. Chin J Integr Med. 2012;18:72–79. doi: 10.1007/s11655-011-0881-3. [DOI] [PubMed] [Google Scholar]
- Bansal P, Paul P, Nayak PG, Pannakal ST, Zou J-H, Laatsch H, et al. Phenolic compounds isolated from Pilea microphylla prevent radiation-induced cellular DNA damage. Acta Pharm Sin B. 2011;1:226–235. doi: 10.1016/j.apsb.2011.10.006. [DOI] [Google Scholar]
- Bhattacharya S. Medicinal plants and natural products in amelioration of arsenic toxicity: a short review. Pharm Biol. 2017;55(1):349–354. doi: 10.1080/13880209.2016.1235207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. doi: 10.1186/s12906-017-1845-6. [DOI] [Google Scholar]
- Gouthamchandra K, Mahmood R, Manjunatha H. Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ Toxicol Pharmacol. 2010;30:11–18. doi: 10.1016/j.etap.2010.03.005. [DOI] [PubMed] [Google Scholar]
- John D. One hundred useful raw drugs of Kani tribe of India. Int J Crude Drug Res. 1984;22:17–39. doi: 10.3109/13880208409070646. [DOI] [Google Scholar]
- Mazumder DNG. Chronic arsenic toxicity human health. Indian J Med Res. 2008;128:436–447. doi: 10.1016/j.kjms.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Renvoize C, Biola A, Pallardy M, Breard J. Apoptosis: identification of dying cells. Cell Biol Toxicol. 1998;14:111–120. doi: 10.1023/A:1007429904664. [DOI] [PubMed] [Google Scholar]
- Saha MR, Alam MA, Akter R, Jahangir R. In-vitro free radical scavenging activity of Ixora coccinea L. Bangladesh J Pharmacol. 2008;3:90–96. doi: 10.3329/bjp.v3i2.838. [DOI] [Google Scholar]
- Sankaranarayanan S, Bamal P, Ramachandran J, Kalaichelvan PT, Deccaraman M, Vijayalakshimi M, et al. Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamilnadu, India. J Med Plants Res. 2010;4:1089–1101. doi: 10.5897/JMPR09.027. [DOI] [Google Scholar]
- Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Singh AP, Goel RK, Tajpreet K. Mechanisms pertaining to arsenic toxicity. Toxicol Int. 2011;18:87–93. doi: 10.4103/0971-6580.84258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaperumal R, Ramya S, Veera Ravi S, Rajasekaran C, Jayakumararaj R. Herbal remedies practiced by Malayali’s to treat skin diseases. Environ Int J Sci Tech. 2009;4:35–44. [Google Scholar]
- Sivarajan VV, Balachandran I. Ayurvedic drugs and their plant sources. New Delhi: Oxford and IBH Publishing Co. Ltd; 1994. p. 347. [Google Scholar]
- Slinkard J, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. doi: 10.12691/jfnr-3-7-7. [DOI] [Google Scholar]
- Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. Study on antioxidant activity of certain plants in Thailand: mechanism of antioxidant action of guava leaf extract. Food Chem. 2007;103:381–388. doi: 10.1016/j.foodchem.2006.07.034. [DOI] [Google Scholar]
- Vadivu N, Jayshree C, Kasthuri K, Rubhini G, Rukmankathan G. Pharmacognostical standardization of leaves of Ixora coccinea Linn. J Pharm Sci Res. 2010;2:164–170. [Google Scholar]
- Wang Z, Zhao Y, Smith E, Goodall GJ, Drew PA, Brabletz T, Yang C. Reversal and prevention of arsenic induced human bronchial epithelial cell malignant transformation by microRNA—200b. Toxicol Sci. 2011;121:110–122. doi: 10.1093/toxsci/kfr029. [DOI] [PMC free article] [PubMed] [Google Scholar]