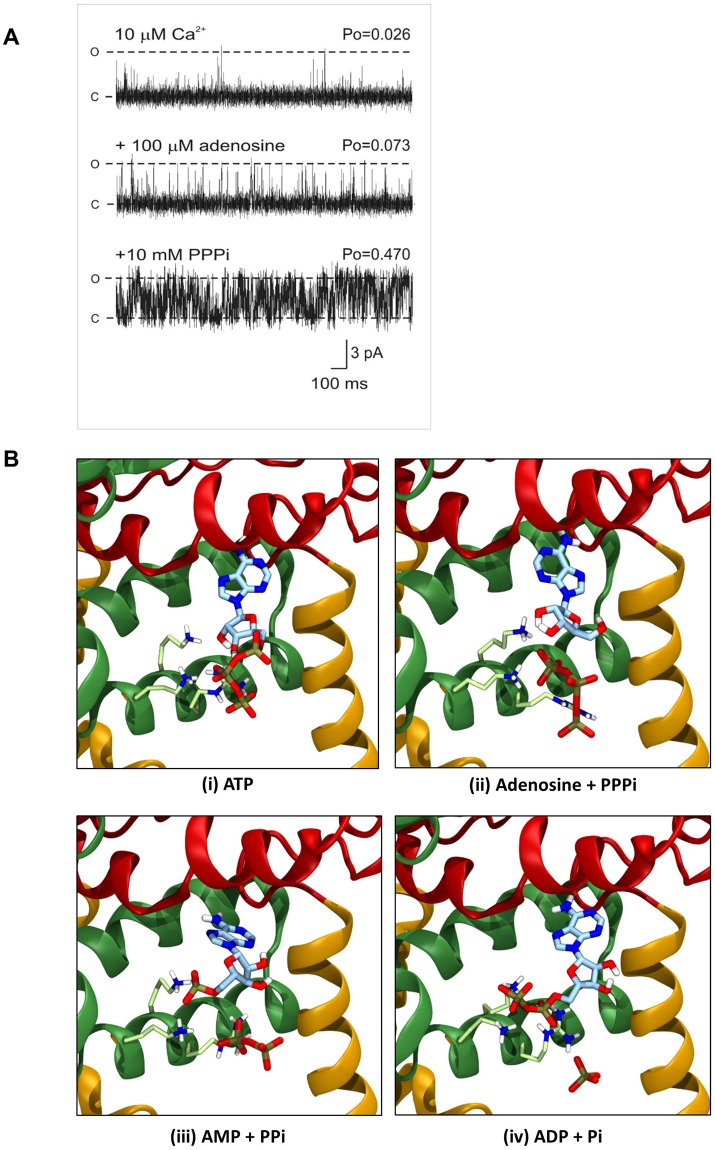
Figure 5.
Effects of adenosine plus PPPi on RyR2 gating and the docking of complementary fragments of ATP. (A) Current fluctuations through a typical single RyR2 channel recording in the presence of 10 μM Ca2+ (top trace), after addition of 100 μM cytosolic adenosine (middle trace) and the subsequent addition of 10 mM cytosolic PPPi in the continued presence of 100 µM adenosine (bottom trace). The holding potential was 0 mV. O and C represent the open and closed channel levels, respectively. (B) The RyR2 ATP binding site with the most ATP-like docking results for (i) ATP, (ii) adenosine and PPPi, (iii) AMP and PPi, and (iv) ADP and Pi. In each case, the nucleoside ligand was docked first, the most ATP-like pose was adopted as part of the protein, and the phosphate ligand subsequently docked into the same site.