Figure 1.
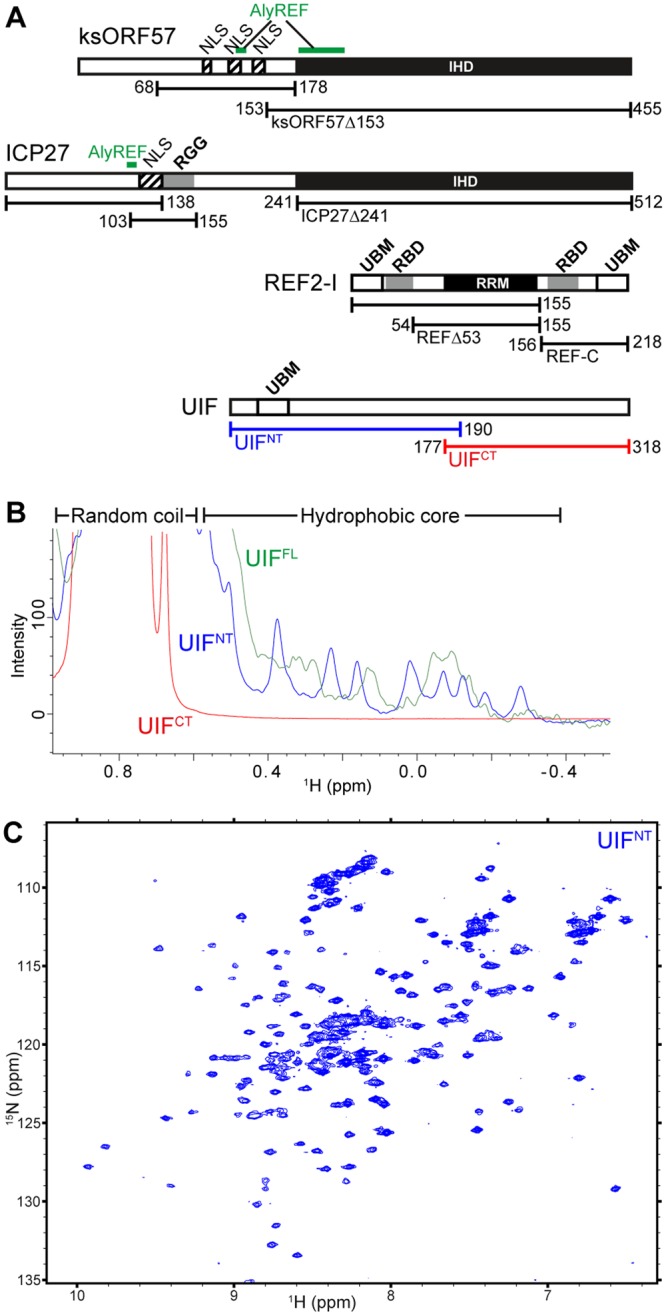
Summary of protein constructs and characterization of the protein folding of UIF by NMR. (A) Schematic of proteins, their domains and functional motifs employed in this study. Known folded domains are indicated as black filled boxes (labeled IHD for ICP27-homology domain, and RRM for RNA-recognition motif). ALYREF binding sites are labeled green and RNA binding motifs grey boxes, nuclear localization sequences (NLS) are shown as hatched boxes, also UAP56-binding motifs (UBM) are labeled. (B) 1D 1H spectra of UIF constructs reveal upfield methyl signals characteristic of a folded globular protein in UIFFL and UIFNT but not UIFCT. (C) 15N HSQC spectrum of UIFNT contains well-dispersed backbone amide signals indicative of a globular folded protein.
