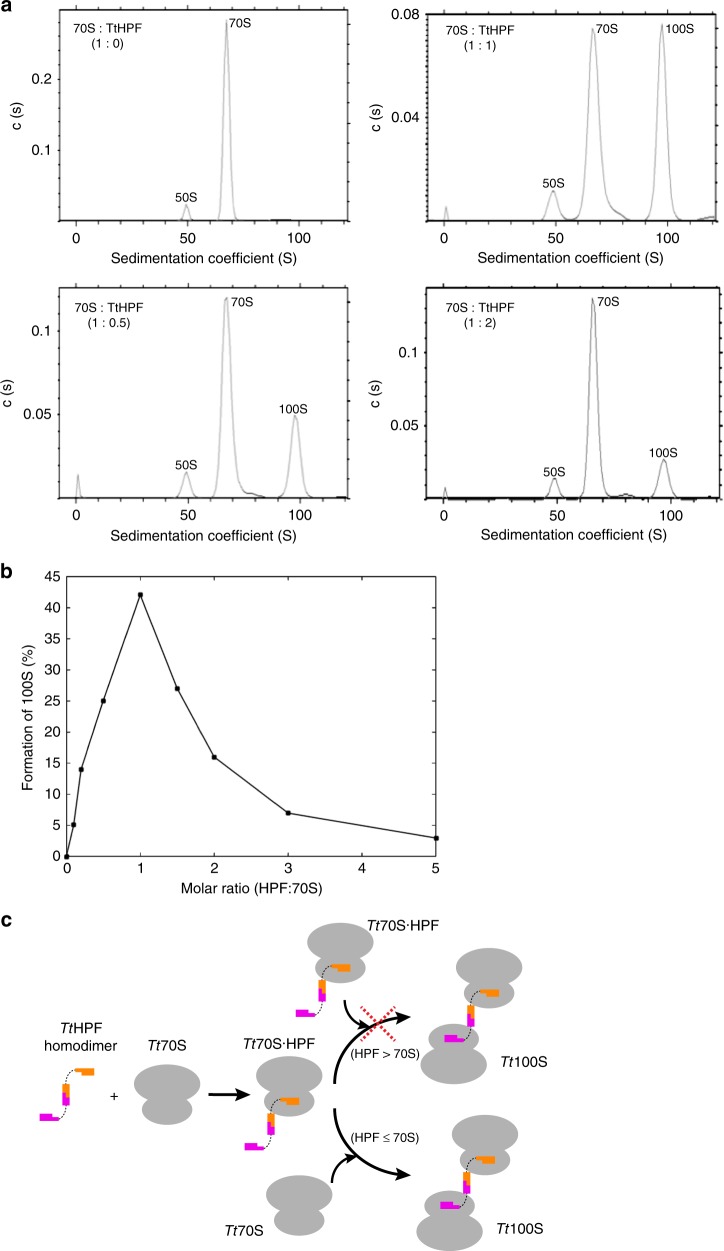
Fig. 1.
Analysis of in vitro TtHPF dependent formation of Tt100S. a Analytical ultracentrifugation sedimentation profiles show 70S ribosome as control (upper left), 70S ribosome mixed with TtHPF in 0.5 times molar ratio (lower left), 70S ribosome mixed with TtHPF in equimolar ratio (upper right) and 70S ribosome mixed with TtHPF in two times molar ratio (lower right). Formation of Tt100S ribosome is evident by the peak at a sedimentation coefficient of 100S. b Graphical representation of Tt100S ribosome formation from all AUC experiments. Formation of Tt100S ribosome by TtHPF is maximal in the case where the molar ratio of TtHPF to Tt70S is 1:1. See also Supplementary Figure 3. c Schematic illustration of TtHPF and Tt70S binding events leading to Tt100S ribosome formation. Binding of one NTD of TtHPF homodimer to Tt70S leads to a complex of Tt70S·TtHPF. In the case of sub- or equimolar ratios of TtHPF and Tt70S, binding of a vacant Tt70S ribosome to the free NTD of the Tt70S·TtHPF complex leads to Tt100S formation. However, in the case of TtHPF being present in excess molar ratios, Tt100S ribosome formation is inhibited because all Tt70S ribosomes bind a TtHPF homodimer