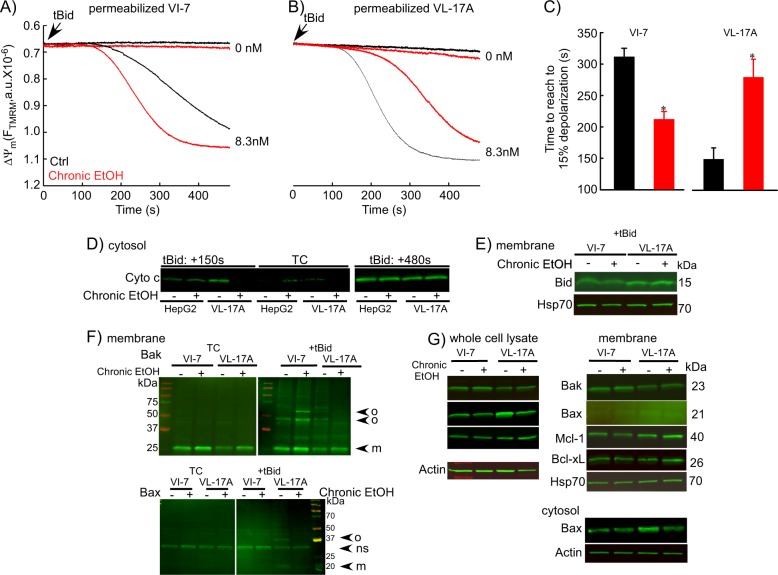
Fig. 9. EtOH sensitizes HepG2 via increased Bak oligomerization but fails to sensitize VL-17A cells to tBid-induced OMMP.
a, b Representative time-course recording of ΔΨm in permeabilized VI-7 a and VL-17A b cell suspension. Arrows show the addition of zero or 8.3 nM tBid. c Bar charts show the average of the time to reach to 15% of the depolarization upon treating the VI-7 and VL-17A cells with 8.3 nM tBid as described in Fig. 8a, b. (*: p < 0.05, n = 4). d Western blot using anti-cyto c antibody for the cytosolic fraction of the EtOH-exposed or non-exposed VI-7 and VL-17A cells. Permeabilized cells were treated with tBid for 150 s (left panel) or 450 s (right panel) and as described in Fig. 6e. TCs are the samples that were not treated with tBid. e Western blot using anti-Bid in the membrane fraction of EtOH-exposed and non-exposed VI-7 and VL-17A cells that were treated with 8.3 nM tBid. Hsp70 was used as loading control. f Western blot using anti-Bak (upper panels) and anti-Bax (lower panels) in the membrane fraction lysates of ethanol exposed and non-exposed VI-7 and VL-17A cells, which were treated with either zero (left panels) or 8.3 nM tBid (right panel). Membrane fractions were separated from cytosol and were treated with BMH (poly-linker) as described in Materials and methods. (O: oligomeric, ns: non-specific band, m: monomeric band) g Western blot of whole cell and membrane fraction lysates or cytosol fraction of the ethanol exposed and non-exposed VI-7 and VL-17A cells using anti-Bak, -Bax, -Mcl-1, -Bcl-xL. Actin or Hsp70 was used as loading control