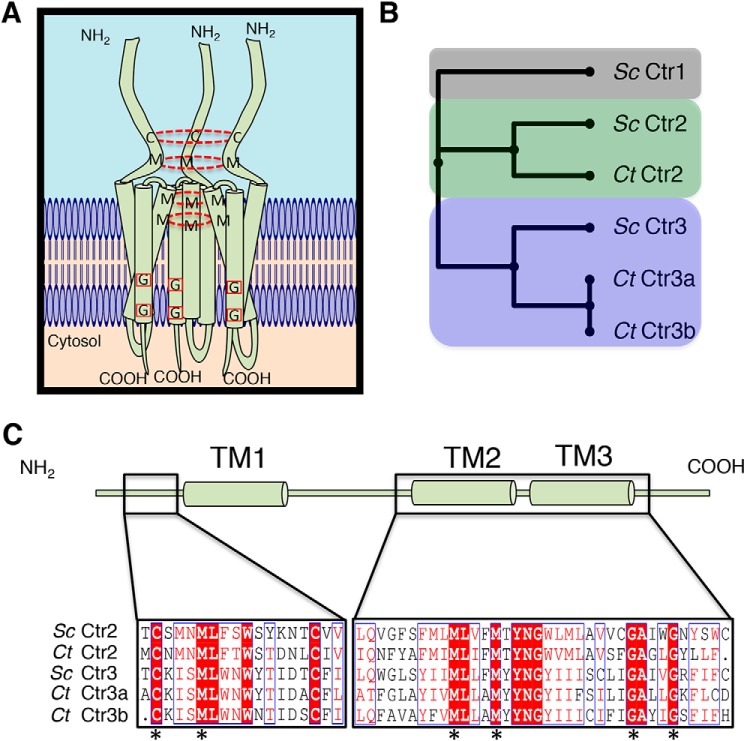
Figure 1.
Bioinformatic analysis identifies C. thermophilum copper transporters. A, model showing a homotrimeric Ctr1/Ctr3 family protein complex with key Cys and Met residues involved in Cu+ transport connected with a dashed red circle and key Gly residues involved in trimer stability via transmembrane helical packing highlighted with a red box. B, putative C. thermophilum Ctr proteins were aligned against characterized S. cerevisiae Ctr proteins with MUSCLE and compiled into a phylogenetic tree. C, C. thermophilum Ctr2 and Ctr3 proteins possess critical functional residues that are hallmarks of Ctr family members. Residues in white type surrounded by red are strictly conserved, residues in red type surrounded by a blue line possess similar biochemical properties, and residues in black type are not conserved. Asterisks indicate strictly conserved residues that are critical for transport activity.