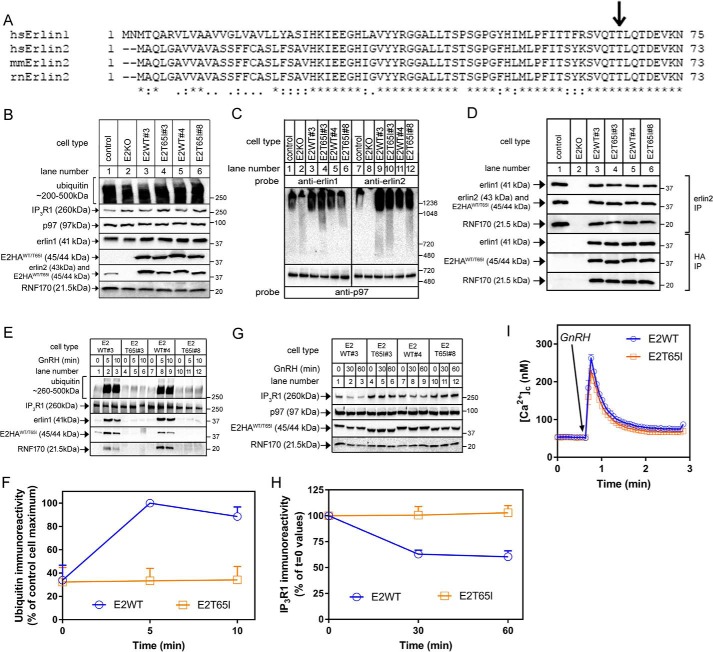
Figure 3.
Effects of the T65I mutation on erlin2 function. A, ClustalO alignment of the N-terminal regions of erlin1 and erlin2 from various species (Homo sapiens, Mus musculus, and Rattus norvegicus). Amino acid identity is indicated by asterisks; colons and periods indicate strongly and weakly conservative differences, respectively. B, lysates from control and E2KO αT3 cells and E2KO cell lines stably expressing E2HAWT or E2HAT65I (two of each; lanes 3–6) were probed in immunoblots for ubiquitin, IP3R1, p97, erlin1, erlin2, HA, and RNF170. C, nondenaturing PAGE of cell lysates probed with anti-erlin1, anti-erlin2, or anti-p97 as a loading control. D, anti-erlin2 or anti-HA immunoprecipitates (IP) from cell lines stably expressing E2HAWT or E2HAT65I probed for the proteins indicated. E and F, cells were treated with 100 nm GnRH for 0, 5, or 10 min; anti-IP3R1 immunoprecipitates were probed in immunoblots for the proteins indicated; and ubiquitin immunoreactivity was quantitated and graphed (n = 2). G and H, cells were treated with 100 nm GnRH for 0, 30, or 60 min; lysates were probed for the proteins indicated; and IP3R1 immunoreactivity was quantitated and graphed (n = 7). I, [Ca2+]c in cell lines stably expressing E2HAWT or E2HAT65I exposed to 100 nm GnRH (n = 11). Error bars represent S.E.