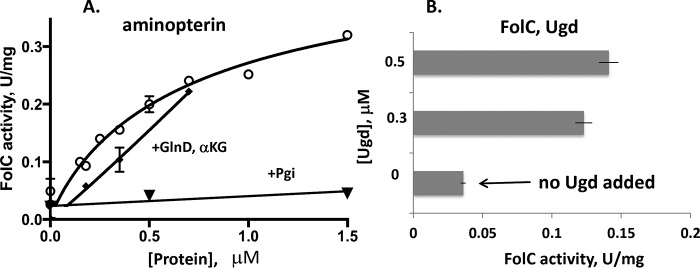
Figure 3.
Allosteric activation of FolC by the GlnD protein. A, activity was measured at 4 mm l-glutamate with 400 μm aminopterin in an assay mixture containing Tris-HCl, pH 8.7, 3 mm DTT, 50 mm KCl, 10 mm MgSO4, and 5 mm ATP in the presence of 0–1.5 μm GlnD (circles) or phosphoglucose isomerase, Pgi (inverted triangles), as a negative control. The α-ketoglutarate (αKG) effect on FolC activity was measured with an l-glutamate concentration of 3 mm and 120 μm aminopterin in the presence of 0–0.7 μm GlnD and αKG (3.5 mm; squares). B, the kinetics were measured as a function of the Ugd (0–0.5 μm) at 1 mm glutamate and an aminopterin concentration of 200 μm. The experiments were repeated two times, and error bars indicate standard deviations.