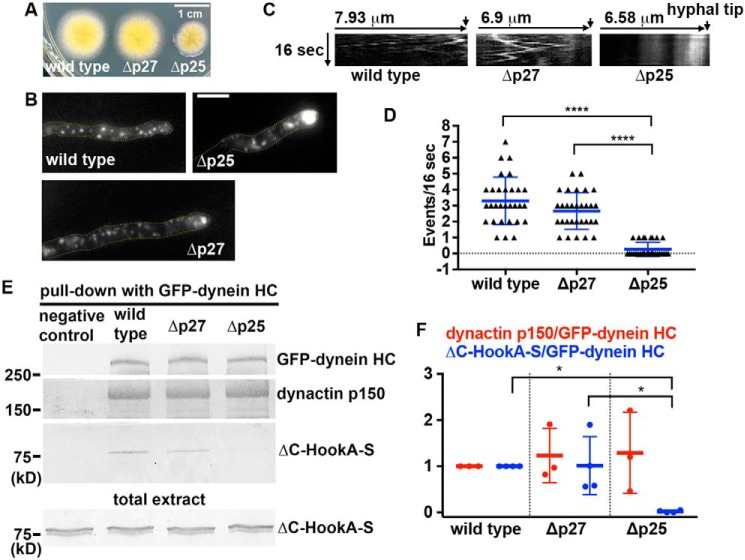
Figure 1.
Analysis on the roles of A. nidulans p27 in early endosome movements and dynein–HookA interaction. A, colony phenotypes of a WT control strain, the Δp27 mutant, and the Δp25 mutant. B, microscopic images showing the distributions of mCherry–RabA-labeled early endosomes in WT and the Δp27 and Δp25 mutant strains. Yellow dotted lines show the hyphal shape. Bar, 5 μm. C, kymographs showing early endosome movements. For each kymograph, position of the hyphal tip is on the right side and indicated by a short arrow (hyphal tip is on the last kymograph). D, frequency of dynein-mediated retrograde early endosome movements in WT, the Δp27 mutant, and the Δp25 mutant. The frequency is defined as the number of mCherry–RabA-positive particles moving away from the hyphal tip in a single hypha within a 16-s period (n = 30 hyphae for each strain). The individual values are shown in a scatter plot together with the mean and S.D. values. **** indicates p < 0.0001 (Kruskal-Wallis test, one-way ANOVA, nonparametric test, unpaired, Prism 7). If p > 0.05, the difference is considered to be nonsignificant and not specifically indicated by any * (this applies to all figures in this paper). E, Western blots showing ΔC-HookA-S and dynactin p150 pulled down with GFP–dynein HC in the WT, Δp25, and Δp27 mutants. The bottom blot shows ΔC-HookA-S signals in total extract of each strain used for the pulldown experiment. F, quantitative analysis on the ratio of pulled-down dynactin p150 to GFP–dynein HC (dynactin p150/GFP–dynein HC, red, n = 3 individual pulldown experiments for each strain) and that of pulled-down ΔC-HookA-S to GFP–dynein HC (ΔC-HookA-S/GFP–dynein HC, blue, n = 4 individual pulldown experiments for each strain). Values of the mutants are relative to the values of the WT controls, which are set at 1. The individual values are shown in a scatter plot together with the mean and S.D. values. * indicates p < 0.05 (one-way ANOVA, unpaired).