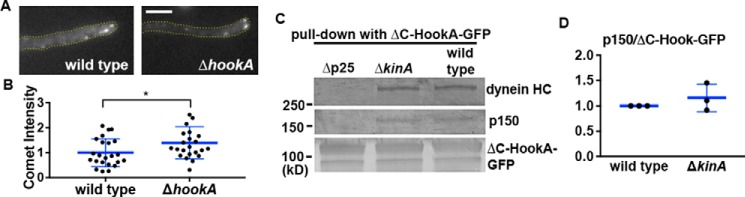
Figure 6.
MT plus-end accumulation of dynactin and the binding of ΔC-HookA to dynein–dynactin are independent of each other. A, microscopic images showing p150–GFP signals in a WT control and the ΔhookA mutant. In both the WT control and the ΔhookA mutant, p150–GFP proteins form comet-like structures near the hyphal tip, representing the MT plus-end accumulation of dynactin. Yellow dotted lines show the hyphal shape. Bar, 5 μm. B, quantitative analysis of the comet intensity in WT and the Δp25 mutant. All the values are relative to the mean value of the WT, which is set at 1 (n = 23 for both strains). The individual values are shown in a scatter plot together with the mean and S.D. values. * indicates p < 0.05 (t test, unpaired). C, Western blots showing that ΔkinA does not apparently affect the interaction between ΔC-HookA–GFP and dynein–dynactin. The Δp25 strain was used as a negative control. D, quantitative analysis on the ratio of the pulled-down p150 to ΔC-HookA–GFP. The ratios of the ΔkinA mutant were normalized to the WT ratios, which were set as 1. The individual values are shown in a scatter plot together with the mean and S.D. values (n = 3 individual pulldown experiments for both strains).