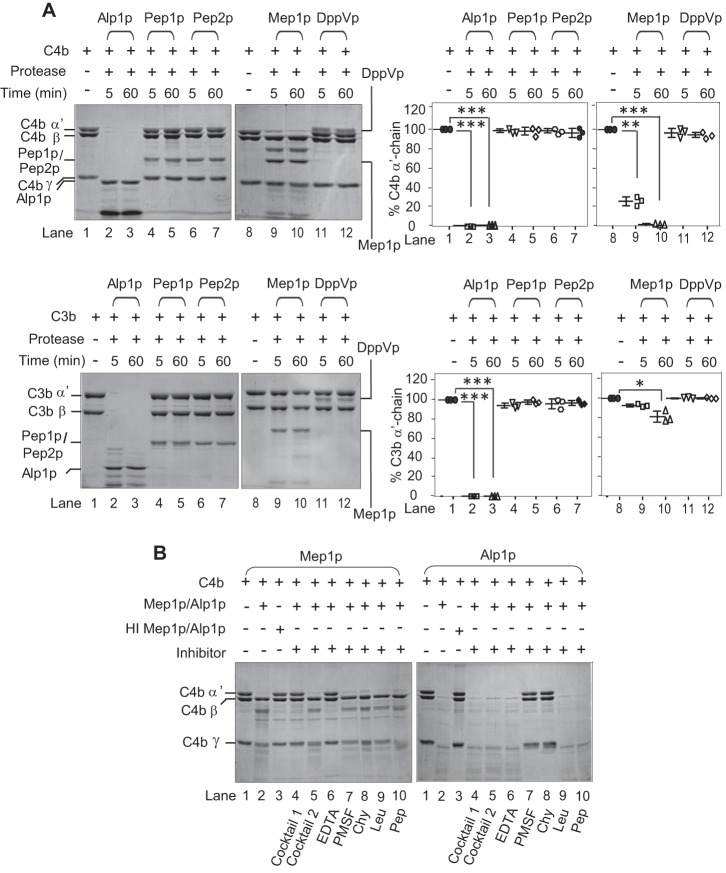
Figure 2.
Proteolytic activity of A. fumigatus proteases toward human complement proteins C4b and C3b. A, proteolytic activity of recombinant proteases (Alp1p, Pep1p, Pep2p, Mep1p, or DppVp) was observed by incubating 1 μg of each of the proteases with 3 μg of C4b (upper left panels) or C3b (lower left panels) for 5 or 60 min in Tris buffer at 37 °C. The cleavage products were visualized by running the samples on 10% SDS-PAGE under reducing conditions and staining them with Coomassie Blue. Among these proteases, only Alp1p and Mep1p showed proteolytic activity toward C4b. The right panels show the % cleavage of α′-chain of C3b and C4b (mean ± S.D. of three experiments; *, p < 0.05; **, p < 0.005; ***, p < 0.0005). B, inhibition of proteolytic activity of Mep1p by various classes of protease inhibitors. Mep1p (0.25 μg) was incubated with C4b (3 μg) in the presence of the indicated inhibitor in Tris buffer for 60 min at 37 °C, and the cleaved fragments were visualized by running the samples on 10% SDS-PAGE and staining with Coomassie Blue. Inhibitors: cocktail 1, complete mini mixture (1×) (Roche Applied Science); cocktail 2, complete mini, EDTA-free (1×) (Roche Applied Science); EDTA (10 mm); PMSF (1 mm); chymostatin (Chy) (100 μg/ml); leupeptin (Leu) (2.5 μg/ml); pepstatin (Pep) (11 μg/ml). The percentage of C4b cleaved was quantitated by densitometric analysis of the α′-chain band and presented as mean ± S.D. of three experiments in Fig. S2. The proteolytic activity of Mep1p for C4b was inhibited by EDTA, whereas that of Alp1p was inhibited by the serine protease inhibitors. Molecular weights: C4b α′-chain, 88,000; C4b β-chain, 75,000; C4b γ-chain, 33,000; C3b α′-chain, 105,000; C3b β-chain, 75,000.