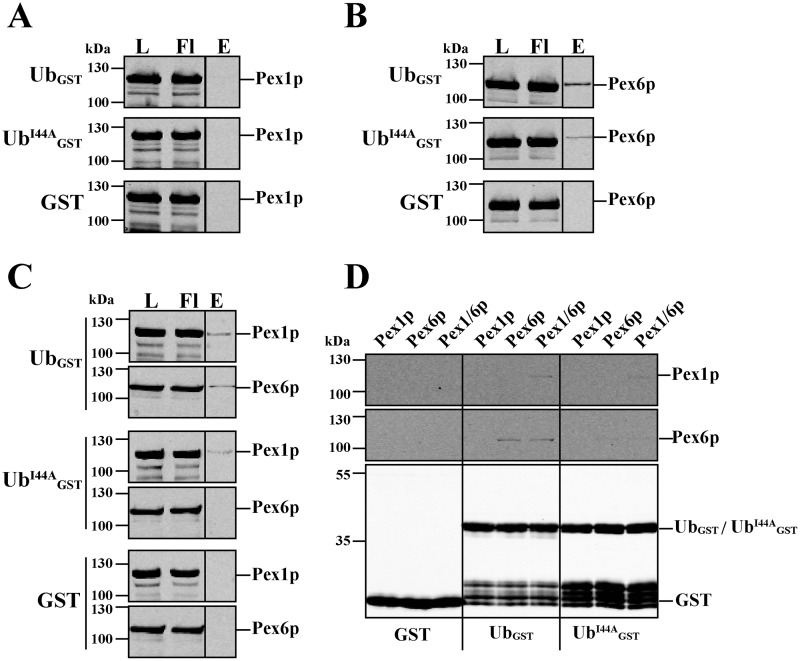
Figure 2.
In vitro pulldown assay of ubiquitin and Pex1p–Pex6p. Recombinant GST and C-terminal GST fusion constructs of WT and mutant (I44A) ubiquitin were combined with purified recombinant HisPex1p (A), HisPex6p (B), or the assembled HisPex1p–HisPex6p complex (C) and loaded onto GSH–agarose. Bound proteins were eluted with buffer containing 50 mm reduced GSH. Equal volumes of load, flow through (Fl), and 10× concentrated eluate fractions were subjected to immunoblot analysis using antibodies against Pex1p, Pex6p, and GST. A comparison of all eluate fractions is shown in D. Pex6p alone and in combination with Pex1p, but not Pex1p alone, did bind ubiquitin. A maximum of 1–2% of loaded AAA proteins could be recovered in the eluate. The I44A mutation reduced binding to Pex6p.