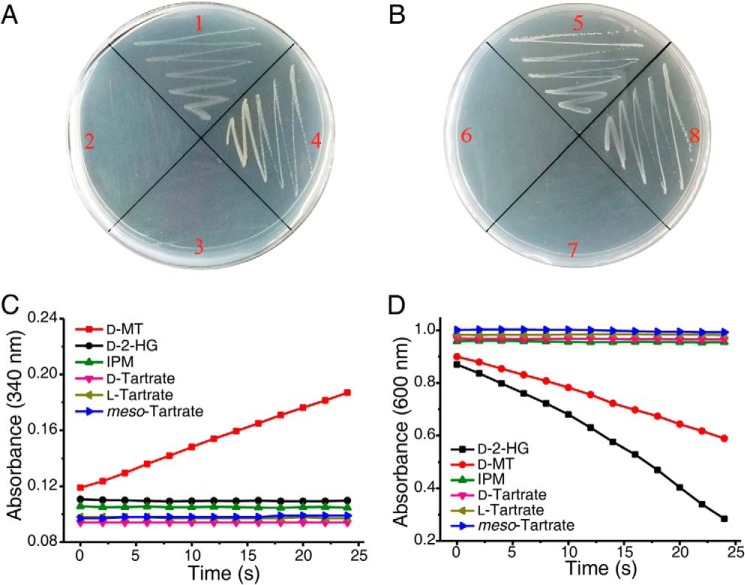
Figure 7.
D2HGDH in P. stutzeri A1501 and d-malate dehydrogenase in E. coli are not mutually replaceable. A and B, growth tests were performed on solid media supplemented with d-malate as the sole carbon source. Quadrant 1, P. stutzeri A1501 WT; quadrant 2, P. stutzeri A1501-Δd2hgdh; quadrant 3, P. stutzeri A1501-Δd2hgdh-dmlA+; quadrant 4, P. stutzeri A1501-Δd2hgdh-d2hgdh+; quadrant 5, E. coli K-12 MG1655 WT; quadrant 6, E. coli K-12 MG1655-ΔdmlA; quadrant 7, E. coli K-12 MG1655-ΔdmlA-d2hgdh+; and quadrant 8, E. coli K-12 MG1655 ΔdmlA-dmlA+. C, dehydrogenation reaction catalyzed by purified recombinant DmlA in E. coli K-12 MG1655 toward different substrates. The activities were measured by detecting the increase of absorbance at 340 nm relating to NADH formation. D, dehydrogenation reaction catalyzed by purified recombinant D2HGDH in P. stutzeri A1501. The activities were measured by detecting the decrease of absorbance at 600 nm relating to DCIP reduction. IPM, isopropylmalate; d-MT, d-malate.