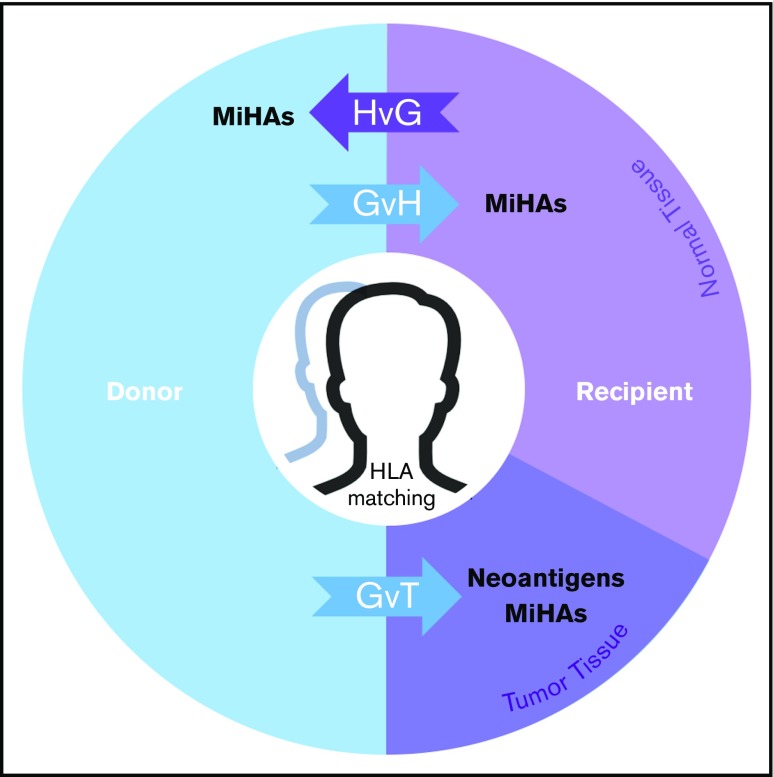
Figure 5.
MiHAs contribute to the therapeutic benefits and adverse effects of allo-HCT. In the donor-to-recipient direction, germline-encoded variant peptides (some of which may be presented by recipient HLA molecules) are expressed on both normal and tumor tissue and thus may contribute to GVT or graft-versus-host (GvH) effects. Tumor-specific somatic mutations that encode immunoreactive neoantigens contribute to GVT. In the recipient-to-donor direction, MiHAs may have host-versus-graft (HvG) effects in various clinical contexts leading to rejection in HCT and solid organ transplant (SOT) or miscarriage in pregnancy. Matching of HLAs reduces alloreactive responses from donor or host immune systems in transplantation settings. With cord blood HCT, HLA matching is usually performed at fewer (6) loci. This model does not fully illustrate the genomic and immunological complexities of graft predominance with multiple unit infusion. With haploidentical pairs, GvH effects in the recipient are controlled nongenetically with prophylaxis. Predictable patterns of germline inheritance determine match rates at HLA (4/8) and MiHAs (50%) with consequent effects on GVT. The presence and therapeutic benefit of neoantigens (because they are not heritable) are predicted to be independent of graft source.