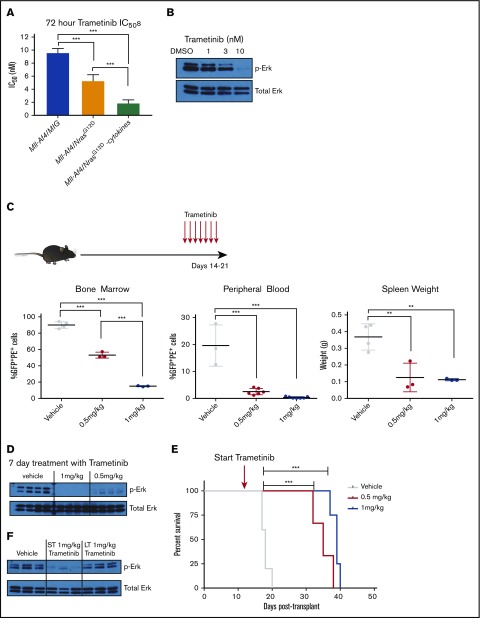
Figure 2.
In vitro and in vivo sensitivity of Mll-Af4/N-RasG12DB-ALLs to MEK inhibition. (A) Mll-Af4/N-RasG12D and Mll-Af4/MIG preleukemic cells were treated with trametinib for 72 hours, and cell viability was measured by 4′,6-diamidino-2-phenylindole staining by flow cytometry. All experiments were conducted in triplicate, and data are represented as a percentage of dimethyl sulfoxide controls. (B) Phospho-Erk and total Erk protein levels of Mll-Af4/N-RasG12D mice treated with trametinib for 24 hours. (C) Leukemic burden in bone marrow, as measured by double-positive cells, white blood cell counts, and spleen weights of mice injected with 1000 secondary Mll-Af4/N-RasG12D leukemic cells and treated in vivo once daily for 7 days, by oral gavage, with vehicle or trametinib (0.5 mg/kg or 1 mg/kg) 14 days after injection (red arrows). (D) Phospho-Erk levels in leukemic bone marrow cells (sorted for tdTomato and GFP double positivity) in mice treated for 7 days with trametinib by oral gavage or vehicle controls. (E) Kaplan-Meier survival plot of prolonged treatment with trametinib. (F) Phospho-Erk levels of leukemic cells from the bone marrow of mice on short-term (ST; 7 days), long-term (LT; ≥20 days), or prolonged treatment with trametinib (1 mg/kg) or from vehicle controls (from Figure 1E). For all in vivo experiments, n ≥ 4 mice per group. Data are representative of ≥3 independent treatment experiments. ***P < .001, **P < .01.